sop for product recall in pharma
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure of recall of marketed product.
2.0 SCOPE
2.1 This SOP is applicable to finished products to be recalled which were manufactured and distributed in:
2.1.1 Domestic market through distributors (C&F), stockiest, retailers and marketing managers (physician sample/clinical test samples).
2.1.2 Export market through distributors.
3.0 RESPONSIBILITY
3.1 Asst. Manager/Executive QA shall be responsible for initiation of product recall to Distribution Head and Marketing Head (domestic and/or export division).
3.2 If product is distributed in domestic market, Distribution Head and Marketing Head are responsible to ensure the immediate recall of unsold stock of batches asked for recall. They are also responsible to ensure the availability of NIL stock certificate from each and every C&F and stockiest.
3.3 If product is distributed in export market, Marketing Head of export division is responsible to ensure the immediate recall of unsold stock of batches asked for recall. They are also responsible to ensure the availability of NIL stock certificate from each and every distributor.
4.0 ACCOUNTABILITY
4.1 Head – Quality Assurance / Chief Executive Officer
5.0 REFERENCE(S)
5.1 In-House
6.0 PROCEDURE:
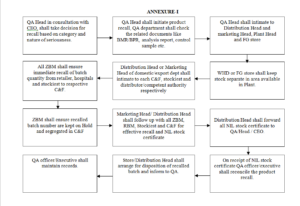
6.1 INITIATION OF PRODUCT RECALL
6.1.1 A finished product shall be recalled from the market due to any of the following reasons:
6.1.1.1 “Not of standard quality” declaration from competent authority i.e. licensing/drug authority.
6.1.1.2 Any abnormal observations on the reference samples or abnormal observation noticed during stability study.
6.1.1.3 As per the investigation report of market complaint.
7.2 RECALL INITIATION AND INTIMATION
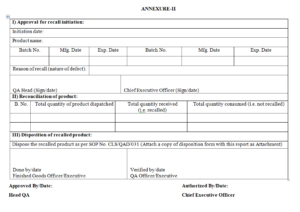
7.2.1 As per any of above mentioned reasons, QA Head shall fill part I of Product Recall Report after authorization from Chief Executive Officer as per Format
7.2.2 QA personnel shall allocate product recall no. as PR/ZZ/NNN
Where,
PR = Product Recall
ZZ = Stand for the last two digits of year
(e.g. 15, if Product recalled in financial year 2015)
NNN = Serial Number
7.2.3 Officer/Executive QA shall record product recall in ‘Product Recall Register’ as per Format (Part A).
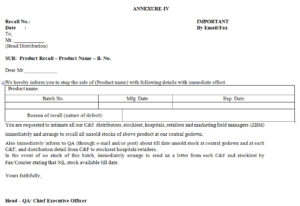
7.2.4 After approval for recall intimation, QA Head / Vice President-Operation shall intimate to Distribution Head (through phone call, email and/or fax) to recall the product as per Format At the same time QA Head shall also inform to Marketing Head (Domestic and/or Export), Chief Executive Officer and FG Store Head through email and/or fax Format
7.2.5 If the batches mentioned in Format are available at production area (i.e. at WIP stage) and/or at finished goods department, manager/head of production and finished goods department respectively shall keep these stock in a separate area of finished goods department with label as “RECALLED PRODUCTS”.
7.2.6 QA shall also ask the following information from Distribution Head along with this intimation:
7.2.6.1 Till date closing stock at godown and C&F.
7.2.6.2 NIL stock certificate from each C&F and stockiest after sending the stock back to factory.
7.2.7 If product was exported to various countries, Marketing Head of Export division shall immediately intimate to recall the product to all distributors and/or competent authorities of all countries through phone call and/or email. For this Marketing Head of Export division must have contact details viz. person name, phone no., fax no., email id address of each and every distributor of all countries.
7.2.8 Distribution Head has to immediately take action accordingly and hence contact details viz. person name, phone no., fax no., email id address of each and every C&F and stockiest must be available with Distribution Head. Following actions to be taken by Distribution Head as soon as product recall intimation received from QA as Format
7.2.8.1 Phone calls to each C&F and ask them to send back all unsold stock to central godown and to send NIL stock certificate in following conditions:
– If no stock is available.
– After sending back all unsold stock to central godown and hence no stock is available.
7.2.8.2 Send letter to each C&F (as Format and stockist (as Format No. ) through register A.D./courier.
7.2.8.3 Inform to QA (through e-mail and/or post) about till date unsold stock at godown and C&F as well as about distribution detail from C&F to stockist.
7.2.8.4 Send letter (as Format ) to ZBM of respective area/territory along with batch distribution detail and unsold stock at C&F as well as distribution details of stockist (i.e. from C&F to stockist).
7.2.8.5 Send letter (as Format No.) to Marketing Head (through e-mail and/or post) about list of ZBM and respective territory intimated.
7.2.9 As soon as recall intimation received from distribution, ZBM with the help of RBM shall ensure the immediate recall of batch quantity from retailers, hospitals and stockist to respective C&F and sending NIL stock certificate from stockist (through fax and/or courier) to godown. ZBM with the help of RBM shall also ensure the recalled batch numbers are kept on ‘hold’ in C&F software as well as physically segregated with appropriate labelling.
7.2.10 All ZBM as well as RBM are also responsible to keep distribution record of physician / clinical test samples and to recall these samples as well.
7.2.11 Marketing Head shall also follow-up to respective ZBM and RBM for effective recall of batch quantity and NIL stock certificate.
7.2.12 Distribution Head shall do constant follow-up with C&F and stockist for effective recall of batch quantity and NIL stock certificate and intimate to ZBM, RBM and/or Marketing Head if necessary.
7.2.13 Distribution Head shall also ensure that all NIL stock certificate shall be forwarded to QA.
Note: In case, the product recall initiated due to critical quality reason (e.g. Product mix up, adverse drug reaction, over dosage, sterility failure) then the batches shall be recalled immediately or within 7 days for both domestic and export supply.
7.3 RECONCILIATION OF RECALLED PRODUCT
7.3.1 On receipt of recalled products, Distribution/Store Head shall ensure that the recalled products are kept in a separate area with label as “RECALLED PRODUCTS”.
7.3.2 After receiving NIL stock certificates from each C&F and stockist, Officer /Executive QA shall record the reconciliation of recall product in part II of Format No. which includes Total quantity of product dispatched, Total quantity received (i.e. recalled) and Total quantity consumed (i.e. not recalled).
7.4 DISPOSITION OF RECALLED PRODUCT
7.4.1 After getting approval from QA department Store, Distribution Head shall arrange for disposition of the recalled product as per SOP No. CLS/QAD/031 and hand over the original copy of disposition form to QA.
7.4.2 Officer/Executive QA shall file filled Format along with a copy of disposition form. He/ She also attach photocopy of Format No. along with disposition form with BMR/BPR of recalled batches.
7.4.3 Officer/Executive QA shall fill up rest of the information in Part B of product recall register.
7.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
Mfg. : Manufacturing Date
Exp. : Expiry Date
C&F : Carrying and Forwarding
ZBM : Zonal Business Manager
RBM : Regional Business Manager
8.0 ANNEXURE
| Annexure No. | Title of Annexure |
| Annexure-I | Flow Chart for Product Recall. |
| Annexure-II | Product Recall Report |
| Annexure-III | Product Recall Register |
| Annexure-IV | Product Recall Intimation to Distribution Head |
| Annexure-V | Product Recall Intimation to C&F (Distributors) |
| Annexure-VI | Product Recall Intimation to Stockiest |