Investigation and Root cause analysis
1.0 OBJECTIVE:
To lay down the procedure for Investigation and Root cause analysis.
2.0 SCOPE:
This procedure is applicable to abc company. facility for investigation and root cause analysis of un explained discrepancies resulting from below listed area but not limited to.
2.1 People
2.2 Process
2.3 Material
2.4 Equipment
2.5 Environment
2.6 Planning
3.0 RESPONSIBILITY:
3.1 QA manager or designee along with related area representative is responsible for investigation and root cause analysis.
3.2 All Employee of QA, QC, Production / Engineering / Warehouse / Human Resources shall be responsible to report the discrepancies.
4.0 ACCOUNTABILITY:
QA Head and concerned department Head shall be accountable that this SOP is implemented as per laid down procedure.
5.0 PROCEDURE:
5.1 Definitions of the key components of the Investigation are listed below
5.1.1 Investigation–A documented analysis conducted to determine root causes(s) for any unexplained discrepancy and the failure.
5.1.2 Root Cause – The identifiable factor(s) based upon objective evidence, which has been determine to be responsible for unexplained discrepancies.
5.1.3 Investigation team: Investigation team includes QA manager or designee along with designated person from concerned department which shall carry out the investigation and root cause analysis as per the defined in SOP.
5.2 Investigations are to be conducted for any unexplained discrepancy as per elements and checklist mentioned below but it is not limited to the followings.
5.2.1 People : The Principal cause of the deviation is an omission or a mistake made by people in the execution of their work.
Elements — Checklist
1.1.1.1 Procedure :- Misunderstanding in the interpretation or execution of a procedure.
1.1.1.2 Training :- Lack or absence of training.
1.1.1.3 Communication :- Lack or incorrect exchange of information.
1.1.1.4 Calculation :- Mistake in the execution of the calculation or data replication, transmission.
1.1.1.5 Accident :- Accident caused by lack of attention or superficiality.
1.1.1.6 Documentation:- Incorrect or incomplete compilation of the documentation.
1.1.1.7 Other :- None of the above, but still due to people.
5.2.2 Process :- The principle cause for the deviation is an issue related to the process (manufacturing, packaging, etc).
| Elements | Checklist | |
| 1.1.1.1 | Planning | Failing to follow the time scheduled for a production phase. |
| 1.1.1.2 | Non Compliance | Process executed being not in compliance with the validated one |
| 1.1.1.3 | Formula | Inability to follow the validated process or one of its phases according to formula. |
| 1.1.1.4 | Yield | All cases in which the yield limits defined in procedures are not met. |
| 1.1.1.5 | Process Parameters | All cases in which in process control checks are out the defined limits. |
| 1.1.1.6 | Documentation | The Documentation related to the process is unclear or difficult to understand. |
| 1.1.1.7 | Registration | The process departures from the limits defined in the Registration Dossier. |
| 1.1.1.8 | Analytical Test | After the process, the product is out of the defined limits. |
| 1.1.1.9 | Other | None of the above, but due to process |
5.2.3 Materials : The Principle reason for the deviation is the non-compliance of any material.
| Elements | Checklist | |
| 1.1.1.1 | Packaging Components | Packaging components supplied by external suppliers, including primary packaging components. |
| 1.1.1.2 | Printed Component | Printed labels, Cartons and leaflets. |
| 1.1.1.3 | Primary ingredients | All the primary ingredients, including the Active Primary Ingredients. |
| 1.1.1.4 | Other | None of the above, but due to materials. |
5.2.4 Equipment : The Principal reason for the deviation is related to machinery used in the production process
| Elements | Checklist | |
| 1.1.1.1 | Breakage or Malfunctioning | Malfunctioning or breakage of any machine or its parts |
| 1.1.1.2 | Unsuitable or absent Machine | Use of an unsuitable machine or absence of the machine or of any
of its parts |
| 1.1.1.3 | Set-up | Difficulties in the set-up phase. |
| 1.1.1.4 | Maintenance | Lack of poor maintenance. |
| 1.1.1.5 | Systems | Departures due to lack or poor information technologies systems (software, PLC, etc.) |
| 1.1.1.6 | Other | None of the above, but due to equipment. |
5.2.5 Environment : The principal reason for the deviation is the environmental conditions, which have affected the quality of the product.
| Elements | Checklist | |
| 1.1.1.1 | Water | Includes all the types of water (drinkable, PW, HPW, WFI, etc.). |
| 1.1.1.2 | Utilities | Includes steam, electricity, compressed air, nitrogen gas, vacuum, venting systems and conditioning (humidity, temperature, overpressure, etc) |
| 1.1.1.3 | Particulate Contamination | Particle limits are exceeded or particulate contamination is found. |
| 1.1.1.4 | Viable Contamination | Deviation because of non compliance to the limit. |
5.2.6 Planned : The Principal reason for the deviation a planned activity, which may affect the quality of the product
| Elements | Checklist | |
| 1.1.1.1 | Equipment or trials assessments | Includes any trials to temporarily replace equipment for Environmental break door other reasons and / or for any environmental. |
| 1.1.1.2 | Procedure, training or documentation | Includes any alternations due to procedural, training or documentation reasons. |
| 1.1.1.3 | Process trial | Includes any trials to temporarily amend process for assessment purposes, may prelude change. |
5.3 An investigation shall be conducted to determine the root cause of any problem. The diagrammatic representation is as follow:
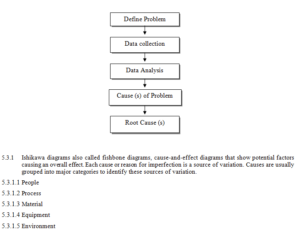

5.4 Brainstorming (for looking at cause and effect and Problem solving)
5.4.1 Brainstorming is a group or individual creativity technique by which efforts are made to find a conclusion for a specific problem by gathering a list of ideas spontaneously contributed by its member(s). Brainstorming is either individual or group.
5.4.2 Individual Brainstorming:
While group brainstorming is often more effective at generating ideas than normal group problem-solving, study after study has shown that when individuals brainstorm on their own, they come up with more ideas (and often better quality ideas) than groups of people who brainstorm together.
Partly this occurs because, in groups, people aren’t always strict in following the rules of brainstorming and bad group behaviors creep in. Mostly, though, this occurs because people are paying so much attention to other people’s ideas that they’re not generating ideas of their own – or they’re forgetting these ideas while they wait for their turn to speak. This is called “blocking”.
When you brainstorm on your own, you’ll tend to produce a wider range of ideas than with group brainstorming – you do not have to worry about other people’s egos or opinions, and can therefore be more freely creative. For example, you might find that an idea you’d be hesitant to bring up in a group session develops into something quite special when you explore it with individual brainstorming. Nor do you have to wait for others to stop speaking before you contribute your own ideas.
You may not, however, develop ideas as fully when you brainstorm on your own, as you do not have the wider experience of other members of a group to help you.
5.4.3 Group Brainstorming
When it works, group brainstorming can be very effective for bringing the full experience and creativity of all members of the group to bear on an issue. When individual group members get stuck with an idea, another member’s creativity and experience can take the idea to the next stage. Group brainstorming can therefore develop ideas in more depth than individual brainstorming.
Another advantage of group brainstorming is that it helps everyone involved to feel that they’ve contributed to the end solution, and it reminds people that other people have creative ideas to offer. What’s more, brainstorming is fun, and it can be great for team-building!
Brainstorming in a group can be risky for individuals. Valuable but strange suggestions may appear stupid at first sight. Because of this, you need to chair sessions tightly so that ideas are not crushed, and so that the usual issues with group problem-solving don’t stifle creativity.
5.5 Five Whys (for looking at cause and effect)
5.5.1 The 5 Whys is an iterative question-asking technique used to explore the cause-and-effect relationships underlying a particular problem. The primary goal of the technique is to determine the root cause of a defect or problem. (The “5” in the name derives from an empirical observation on the number of iterations typically required to resolve the problem.)
The following example demonstrates the basic process:
The vehicle will not start. (The problem).
1. Why? – The battery is dead. (first why)
2. Why? – The alternator is not functioning. (second why)
3. Why? – The alternator belt has broken. (third why)
4. Why? – The alternator belt was well beyond its useful service life and not replaced. (fourth why)
5. Why? – The vehicle was not maintained according to the recommended service schedule. (fifth why, a root cause)
6. Why? – Replacement parts are not available because of the extreme age of the vehicle. (sixth why, optional footnote)
Start maintaining the vehicle according to the recommended service schedule. (possible 5th Why solution)
Purchase a different vehicle that is maintainable. (possible 6th Why solution)
The questioning for this example could be taken further to a sixth, seventh, or higher level: the “five” in 5 Whys is not gospel, but five iterations of asking why is generally sufficient to get to a root cause. The key is to encourage the trouble-shooter to avoid assumptions and logic traps and instead trace the chain of causality in direct increments from the effect through any layers of abstraction to a root cause that still has some connection to the original problem. Note that in this example the fifth why suggests a broken process or an alterable behavior, which is typical of reaching the root-cause level.
5.6 All the investigation and root cause analysis is followed by CAPA as per SOP.
6.0 ABBREVIATION:
| S. No. | Abbreviations used | Full form of Abbreviation used |
| 1.0 | SOP | Standard operating procedure |
| 2.0 | QA | Quality Assurance |
| 3.0 | QC | Quality control |
| 3.0 | PW | Purified water |
| 4.0 | HPW | Hot Purified water |
| 5.0 | WFI | Water for injection |
| 6.0 | PLC | Programmable logical control |
7.0 ATTACHMENTS (ANNEXES): NIL
8.0 REFERENCE :
| S. No. | Reference Title | |
| 1.0 | Pharmaceutical Quality System Q10
|
|
sop for Calibration and Maintenance of Laboratory Instruments and Equipment
Disposal of Residual Sample or Left Over Material
sop for for Laboratory Incident
standard operating procedure temperature monitoring
sop for operation of infrared moisture balance
sop for preparation of mobile phase
sop for Preparation and Issuance of Analysis protocol standard
sop of placebo and impurity stock solutions
sop for disposal of residual sample
sop for handling of pharmacopoeial changes
sop for procedure for operation of ultrasonic cleaner
difference between UPLC and HPLC
sop for for Emergency Eyewash and Shower
sop for operation and calibration of total organic carbon analyzers
sop for operation of cobb tester
sop for Operation and calibration of atomic absorption spectrophotometer
sop for Operation and calibration of gas liquid chromatograph
sop for operation of humidity oven
sop for operation and calibration of serological water bath
sop for monitoring of drain trap
sop for destruction of analytical samples after testing and control samples
sop for destruction of used chemicals
Sop for Operation of suction pump
sop for Operation and calibration uv cabinet
sop for Operation and calibration of bulk density apparatus
sop for operation and calibration of shore hardness tester
sop for operation of rub proofness tester
sop for monitoring of purified water
sop for Retesting of packaging materials
sop for Retesting and resampling of raw materials
sop for Control of issuance of record of analysis green sheets
sop for Control of computer passwords
sop for sampling of packaging materials PM
sop for sampling of sterile raw material
sop for sampling of intermediates and finished products
sop for operation and calibration of friability test apparatus
sop for approval and rejection of packaging materials