master formula record deflazacort suspension
| Product Code | ||
| Product (Brand) Name | Deflazacort Suspension | |
| Generic Name | Deflazacort Suspension | |
| Label Claim | Each 5 ml contains | |
| Deflazacort | 6 mg | |
| Excipients | q. s. | |
| Color | As per approved color | |
| Primary Pack Description | Filled in Amber color pet bottles. | |
| Fill Volume | 30 ml , 60 ml | |
| Product Description | Yellowish oral suspension filled in Amber color pet bottles. | |
| Shelf-life | 24 Months | |
| Storage Condition | Store in cool, dry & dark place. | |
2.0. TABLE OF CONTENT :
| Sr. No. | CONTENTS | Page No. |
| 1.0. | PRODUCT INFORMATION | |
| 2.0 | TABLE OF CONTENT | |
| 3.0 | FLOW-CHART FOR FORMULATION | |
| 4.0 | BILL OF RAW MATERIAL & CALCULATION | |
| 5.0 | BATCH MANUFACTURING PROCESS | |
| 6.0 | YIELD AND ACCOUNTABILITY | |
| 7.0 | PRECAUTION |
4.0. BILL OF RAW MATERIAL :
| Sr.
No. |
Ingredients | Pharmacopoeial
Status |
Qty in
(Kg) |
Overages
(%) |
Qty/batch
(Kg) |
| 1.0 | Deflazacort * | 2.400 | – | 2.400 | |
| 2.0 | Sodium Benzoate | 1.000 | – | 1.000 | |
| 3.0 | Methyl Paraben Sodium | 1.000 | – | 1.000 | |
| 4.0 | Propyl Paraben Sodium | 0.500 | – | 0.500 | |
| 5.0 | Xanthan Gum | 8.000 | – | 8.000 | |
| 6.0 | Areosil | 7.000 | – | 7.000 | |
| 7.0 | Sucrose | 500.000 | – | 500.000 | |
| 8.0 | Aspartame | 2.000 | – | 2.000 | |
| 9.0 | Bronopol | 0.400 | – | 0.400 | |
| 10.0 | Tween-80 | 2.000 | – | 2.000 | |
| 11.0 | Sodium Saccharin | 2.000 | – | 2.000 | |
| 12.0 | Flavour Mango | 6.000 Ltr. | – | 6.000 Ltr. | |
| 13.0 | Color Tartrazine | 0.040 | – | 0.040 |

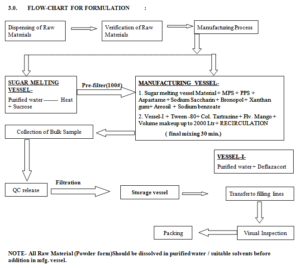
5.0. BATCH MANUFACTURING PROCESS :
5.1 SUGAR SYRUP PREPARATION-
5.1.1 Take the Line clearance from IPQA.
5.1.2 Take the approx. 300 liter of purified water in Sugar Syrup manufacturing vessel & heat it up to 80 ºC then add 500.000 Kg sucrose through vacuum transfer system in Sugar Syrup manufacturing vessel .All the activity under continuous stirring till the sugar syrup base preparation .
5.1.3 After completion of sugar syrup base preparation , cool the solution below 40 ºC then transfer the solution to manufacturing vessel through pre-filter (containing 100 # S.S. Sieve ).
5.2 BULK MANUFACTURING PROCESS-
5.2.1 Take the Line clearance from IPQA.
5.2.2 Take approx. 5.000 Ltr. Purified water and dissolve the 1.000 Kg Methyl paraben sodium & add to manufacturing vessel & mix for 5 min.
5.2.3 Take approx. 5.000 Ltr. Purified water and dissolve the 0.500 Kg Propyl paraben sodium & add to manufacturing vessel & mix for 5 min.
5.2.4 Take approx. 5.000 Ltr. Purified water and dissolve the 1.000 Kg Sodium Benzoate & add to manufacturing vessel & mix for 5 min.
5.2.5 Take approx. 10.000 Ltr. Purified water and dissolve the 2.000 Kg Sodium Saccharin & add to manufacturing vessel & mix for 5 min.
5.2.6 Take approx. 5.000 Ltr. Purified water and dissolve the 0.400 Kg Bronopol & add to manufacturing vessel & mix for 5 min.
5.2.7 Take approx. 10.000 Ltr. Purified water and dissolve the 2.000 Kg Aspartame & add to manufacturing vessel & mix for 5 min.
5.2.8 Take 8.000 Kg Xanthan Gum and add to manufacturing vessel & mix for 20 min.
5.2.9 Take 7.000 Kg Areosil & add to manufacturing vessel & mix for 20 min.
5.2.10 Take approx. 15.000 Ltr. Purified water and dissolve 2.400 Kg Deflazacort, mix it for 20 min. ,then transfer it manufacturing tank& mix for 10 min.
5.2.11 Take approx. 10.000 Ltr. Purified water and dissolve 0.040 Kg color Tartrazine & add it to manufacturing vessel in and mix for 10 min.
5.2.12 Take 6.000 Ltr. Flavour Mango and add it to manufacturing vessel & mix for 10 min.
5.2.13 Take 2.000 Kg Tween-80 in vessel and add it to manufacturing vessel & mix for 10 min.
5.3 pH ADJUSTMENT-
5.3.1 After completion of activity, Check the pH of bulk between 4.0 to 6.0
5.4 VOLUME MAKEUP-
5.4.1 Makeup the volume up to 2000 liters with Purified Water and mix for 30 min.
5.5 RECIRCULATION-
5.5.1 Recirculate the bulk from top to bottom through Homogenizer for 60 min.
5.5.2 Intimate to IPQA to collect the bulk sample .
5.6 FILTRATION-
5.6.1 Filter the final bulk to storage vessel through Homogenizer .
6.0. YIELD AND ACCOUNTABILITY :
6.1 After completion of filtration of bulk, calculate the % yield is between 98 – 100 % .
7.0. PRECAUTION :
7.1 Always wear nose mask & hand gloves before starting the manufacturing process.
7.2 Ensure the Environmental conditions is within limits-
7.2.1. Temperature – NMT 27 ºC
master formula record etofylline and theophylline injection
master formula record l-carnitine injection
master formula record piroxicam injection
master formula record ranitidine injection
master formula record tranexamic acid injection
master formula record tranexamic acid injection
master formula record cetirizine syrup
MFR of sucralfate oral suspension
Master formula record Quinine Sulphate Suspension
MFR of Paracetamol Phenylephrine Hcl Chlorpheniramine Maleate Drops
master formula record paracetamol oral suspension
master formula record ethamsylate 250 mg injection 2 ml
master formula record ofloxacin oral suspension
master formula record Fluconazole Cream
master formula record ondansetron oral solution
MFR of hydroxyzine hydrochloride oral solution
master formula record vitamin D3 drops
master formula record furazolidone oral suspension
MFR of Clobetasol Propionate cream
master formula record Povidone iodine ointment 5%
MFR of Magaldrate Simethicone and Oxetacaine Suspension
master formula record albendazole oral suspension
master formula record ondansetron injection
master formula record nandrolone decanoate injection
MFR of diclofenac sodium injection
master formula record deflazacort suspension