procedure for checking the correctness of the computerised formulation order issue system
1.0 OBJECTIVE
To lay down the procedure for checking correctness of the computerized formulation order issue system.
2.0 RESPONSIBILITY
Production Planning / Quality Control officer
3.0 ACCOUNTABILITY
Production Planning Manager / Quality Assurance Manager
4.0 PROCEDURE
4.1 Print computerized Formulation Order.
4.2 Check the Formulation Order for
i. Manufacturing Date
ii. Expiry Date
iii. Master Formula
iv. “First in First out” system
4.2.1 Manufacturing Date
Check the formulation order for the manufacturing date.
For example: –
The formulation order printed in the month of Jan’2021 shall have the manufacturing date
as “Jan 2021”.
4.2.2 Expiry Date
Check the expiry date of active Ingredient(s), against Analytical Report Number (AR. No.)
Mentioned in the formulation order. Check the correctness by comparing with expiry date mentioned in COA.
Following guideline shall be followed while assigning Expiry for the product.
Domestic Products
The expiry date of the Finished Product shall be as per the expiry date of the product or
expiry date of its active Ingredient (s), whichever is earlier. Products having multiple
active ingredient(s) shall have expiry of Raw Material having the least expiry.
Export Products
The expiry date of the Finished Product shall be as per the Shelf life assigned in Master Formula.
4.3 MASTER FORMULA
4.3.1 Check the Formulation Order against the master formula issued by Product Development Laboratory for following.
i. Code No.
ii. Ingredient(s)
iii. Batch Quantity
iv. Actual Quantity Issued
4.3.2 Calculation of active Ingredient(s) and Filler
Collect potency and moisture data of the active ingredients issued in the formulation order by referring to the Certificate of Analysis from Quality Assurance and check the same with the formulation order.
Calculate the actual quantity of active ingredients to be issued by referring to the formula specified in the Master Formula given by Product Development Laboratory and the quantity of the Filler.
Check the correctness of the quantity by comparing with quantity mentioned in Formulation order.
4.4 “First in-First out” system
Check the “First in – First out” System for any two ingredients issued in Formulation order.
4.4.1 In case of active ingredients, Check for the material in stock having less expiry than that of the issued material. If there is no stock of material having less expiry, the “First out” system shall be followed.
4.4.2 After taking out the Formulation Order, check Analytical Report Number wise stock on computer screen. In case of other ingredients, If there is no stock of previous Analytical Report Number in the system, the “First in-First out” principle shall be considered to be followed.
5.0 In case of any discrepancy, a team comprising of
i. Production Planning Manager
ii. Quality Assurance Manager
iii. Manager Infotech
iv. General Manager Works
Shall investigate the matter and take corrective action. The investigation report shall be documented.
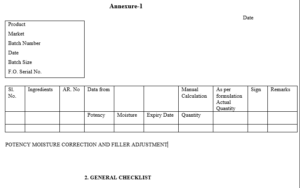
Details of the investigation shall be documented as per Annexure-1.
6.0 ABBREVIATIONS :
AR No. = Analytical Reference Number
COA = Certificate of analysis
7.0 REFERENCES
Annexure I – Investigation report.
| ITEM | CHECKED | SIGN
|
| A. Manufacturing Date
B. Expiry Date |
DONE BY CHECKED BY