Procedure for Microbiologist and QC Chemist Qualification
OBJECTIVE :
1.1 To describe the procedure for qualification of analyst. The purpose of this exercise is to ensure
that the analyst is qualified for Microbial Limit Test and Sterility and Bacterial Endotoxin Test prior
performing the tests on regular basis.
1.2 To lay down a procedure for the qualification QC Analyst.
2.0 SCOPE:
This procedure is applicable for all analyst working in QC and microbiology department
3.0 RESPONSIBILITY:
Microbiologist – Quality control microbiology.
Chemist – Quality control.
4.0 ACCOUNTABILITY :
4.1 Head – QC
5.0 PROCEDURE:
For Microbiology
5.1 Microbial Limit Test Qualification
5.1.1 Manager QC should select three recently approved batches of the same product.
5.1.2 Analyst should analyze the samples of three batches individually.
5.1.3 Analyst should follow the SOP for Microbial Limit Test.
5.1.4 The results should be compared against the previous results.
5.2 Sterility Test Qualification
5.2.1 Manager QC should select three recently approved batches of the same product.
5.2.2 Analyst should analyze the samples of three batches individually.
5.2.3 Analyst should follow the SOP for sterility test.
5.2.4 The results should be compared against the previous results.
5.3 Bacterial Endotoxin Test Qualification
5.3.1 Manager QC should select three recently approved batches of the same product.
5.3.2 Analyst should analyze the samples of three batches individually.
5.3.3 Analyst should follow the SOP for Bacterial Endotoxin test.
5.3.4 The results should be compared against the previous results.
5.4 For Quality Control
5.4.1 Samples of known analytical values shall be identified by the Quality Control Manager.
5.4.2 The analytical value of sample along with acceptable limit. A.R. No. shall be recorded by Quality Control Manager in a register
maintained for this purpose.
5.4.3 All the coded samples shall be kept in sealed vials at 2-80C or as per sample requirement.
5.4.4 The coded samples along with necessary information required for analysis shall be disclosed to the analyst.
5.4.5 Materials already approved by QC laboratory or supplier test report (traceable to authentic testing agency) will be taken as testing material for routine validation exercise.
5.4.6 The validation shall include one or more of the following methods of analysis:
1. HPLC
2. UV Spectrophotometer
3. Titrimetry
5.4.7 The results of analyst shall be checked for QC. Executive compliance and compared with expected values.
5.4.8 Materials Under test with quality monograph (Standard test procedure) will provided to each analyst.
5.4.9 No repeat of test will be allowed to the analyst.
5.5.0 New analyst shall be validated within one year of joining.
5.5.1 After completion of test the QC Manager will check the test result
6.0 ABBREVIATION
| QA | Quality Assurance |
| QC | Quality Control |
| NO. | Number |
| SOP | Standard Operating Procedure |
| HPLC | High Pressure Liquid Chromatography |
| UV | Ultra Violet |
7.0 ATTACHMENTS (ANNEXURE):
ANNEXURE – I Microbial Limit Test Qualification
ANNEXURE – II Sterility Test Qualification
ANNEXURE – III Bacterial Endotoxin Test Qualification
ANNEXURE – IV Analysis Qualification Report
8.0 REFERENCES:
| Sr. No. | Reference Title |
| 01 | In House |
ANNEXURE – I Microbial Limit Test Qualification
MICROBIAL LIMIT TEST QUALIFICATION
| Product Name | Report No. | ||
| Batch No. | Mfg by | ||
| Batch Size | Supp by | ||
| Mfg date | Date of Testing | ||
| Exp date | Date of Completed | ||
| Date of Receipt | Sample Qty |
Media Preparation Details For:-
| Media Name | Soyabean Casein digest Agar | Sabourauds Chloroamphenicol Agar |
| Batch No. |
Testing Details:
Date of Testing: Date of Completion:
| Observations | Plate-I | Plate-II | Mean | Limit | Sign./Date |
| Bacterial Count | NMT | ||||
| Fungal Count | NMT |
Pathogens:
| Testing Parameters | Media Name | Date of Preparation | Media Used Before | Results |
| E. coli | SCDM/Mac Conkey Broth | |||
| Salmonella | SCDM/RVSE Broth | |||
| S. aureus | SCDM/Mannitol salt agar | |||
| P. Aeruginosa | SCDM/Cetrimide agar | |||
| Shigella | SCDM/GN Broth | |||
| Bile Tolrent Gram Negative Bacteria | SCDM/EEM Broth / VRBG Agar |
Media Positive Control:
| Microbial cultures | Bacillus subtilis | Candida albicans | E.coli | Salmonella | Shigella | P. aeruginosa | S. aureus | Observations By |
| Media for Inoculation | SCDA | SDA | MacConkey broth & Agar | RVSE Broth / XLDA | GN Broth/ XLDA | Cetrimide Agar | Mannitol salt Agar | |
| Days
1st 2nd 3rd 4th 5th |
Media Negative control – No Growth / Growth Observed.
Inference: The above test sample Complies/Does not comply as per the standard specifications of IP/BP/USP/IH ….
Comments: This analyst is validated /not validated for routine analysis.
Microbiologist Reviewed By Approved By
Approved By /Date:-
ANNEXURE – II Sterility Test Qualification
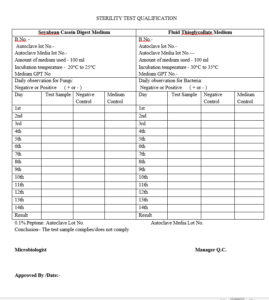
ANNEXURE – III Bacterial Endotoxin Test Qualification
BACTERIAL ENDOTOXIN TEST QUALIFICATION
Name Of Analyst:
Reagent Information:
| Reagent | Lot NO. | Reconstitution Date | Expiry |
| LAL EU/ml | |||
| CSE EU/ml | |||
| LAL Reagent Water |
Control Standard Endotoxin Preparation:
| CONTROL STANDARD ENDOTOXIN SERIES | ||
| Conc. | LRW | CSE |
Test Data and Results:
Date:
Temperature: Start ______ End________
Time: Start______ End________
| CONTROL STANDARD ENDOTOXIN SERIES | |||||
| SERIES | OBSERVATION | END POINT(e) Log Value | |||
| Tube-1 | |||||
| Tube-2 | |||||
| Tube-3 | |||||
| Tube-4 | |||||
| MEAN (Σe/f) | |||||
Geometric Mean:
GM of Endpoints= Antilog ((Σe/f)
Comments: ________________________________________________________________
________________________________________________________________
Reviewed By:
Analyst Name: Product Previous Testing Status:
Product : Date of Testing:
Testing Date: Analyzed by:
Batch No. : Results Status
Potency :
Endotoxin Limit:
MVD:
Tested At :
Test Dilution :
Reagent Information:
| REAGENT | LOT NO. | RECONSTITUTION DATE | EXPIRY |
| LAL EU/ml | |||
| CSE EU/ml | |||
| LAL REAGENT WATER |
Temperature: Start ______ End________
Time: Start______ End________
| Test Dilutions/Concentrations | NPC | PPC | ||
Positive: Gel Formation Negative: No Gel Formation
Analyzed by:
Date:
Conclusion: _________________________________________________________________
_________________________________________________________________
__________________________________________________________________
Reviwed by: Checked By
ANNEXURE – IV Analysis Qualification Report
Name Of Analyst :…………………………….
Test Parameter :……………………………….
Name Of Sample :…………………………….
Designation :………………………………….
| Ar.No. | Batch No. | Test Parameter | Result | Limit | Remark |
COMMENTS………………………………………………………………………………………………………………………………
RESULT CHECKED BY DATE APPROVED BY
APPROVED BY /DATE