sop for annual product quality review APQR
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure for procedure for prepare annual product quality review.
2.0 SCOPE
2.1 This SOP is applicable for prepare annual product quality review of finished product manufactured
3.0 RESPONSIBILITY
3.1 Officer –Quality Assurance – Prepare the SOP and follow-up the SOP accordingly.
3.2 Asst. Manager –Quality Assurance – Provide the support to the implementation of SOP and maintained the records.
4.0 ACCOUNTABILITY
4.1 Head –Quality Assurance / Chief Executive Officer
5.0 REFERENCE(S)
5.1 In-House
6.0 PROCEDURE:
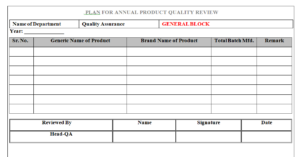
6.1 The Annual Product Quality Review shall be prepared by QA personnel as per APQR plan as mentioned in Format No
6.2 APQR plan shall be prepared for all batches of selected products, manufactured in a year from January to December, in the month of January of next year.
6.3 Product to be selected for preparation of APQR which manufactured at least 5 batches in a year, in case 5 batches are not manufactured in a year then combine with consecutive two years batches.
6.4 Selected batches shall be review for quality review in the following aspects:
6.4.1 Review of the Product Licenses:
6.4.1.1 The company’s license for manufacturing of the selected product has to be verified for the validity. The report should include the license date for the product.
6.4.2 Batch Record Review:
6.4.2.1 The batch record has to be checked for the compliance of the current SOP and specifications.
6.4.2.2 The batch record of the selected batches has to be verified with the current Master copy of the master formula processing and packaging instructions.
6.4.2.3 Any review has been implemented in the MFR should to be reflected in the annual product quality review.
6.4.2.4 If any change controls / deviations incorporated has to be reviewed. The impact of the change on to the quality of the product has to be reviewed in detail with respect to validation and stability. The report should also reflect the change control / deviation control number as reference. The relevant documents should be reviewed for the amendment of obsolete procedure and introduction of current changed procedure.
6.4.2.5 The batch manufacturing record should be observed for any critical discrepancies or non-conformities, and corrective action taken for the same, to ensure that the above had no effect on product quality and safety.
6.4.2.6 Review any reprocessed or reworked batches critically. The annual product quality review should contain the reason for reprocessing or reworking, the corrective action taken and the preventive measures framed to avoid recurrence. Related documents of reprocessing or reworking should be reviewed for their completeness and authorization.
6.4.2.7 The selected batches yield has to be reviewed, stage wise and of the final product, to ensure that they are within the specified limits mentioned in the batch record. If any deficiencies are observed, their comments / reason for the same have to be reviewed. The action taken to avoid the recurrence should be highlighted in annual product quality review. A trend chart for the yield should be prepared.
6.4.3 Review of Analytical Report
6.4.3.1 Review the analytical reports of the selected batches, for the compliance with the current specification of analytical method.
6.4.3.2 The analytical reports of blend, bulk and finished product should be reviewed, and a trend chart for the same to be produced.
6.4.3.3 Review the analytical report of the raw material and packing material used for these selected batches along with current specification. A trend for the same to be prepared.
6.4.3.4 Review out of specification results, which have occurred in the selected batches (RM / PM / Blend / Bulk / FG) reason for the results, re-testing reports and their compliances along with the action taken to avoid recurrence
6.4.3.5 If any new vendor is introduced, the vendor approval form has to be reviewed along with the stability studies of the batches, which has to be reported in annual product quality review.
6.4.3.6 Review method validation protocol and reports if any new method of analysis is introduced.
6.4.3.7 Review the instrument validation and calibration status, their machine log and any maintenance activity during the analysis of the batches under annual product quality review.
6.4.4 Review of the Quality Review Report.
6.4.4.1 Review the current SOP on quality review.
6.4.4.2 Review the quality review reports of the batches under review and mention any discrepancies if observed during the review, reason for the same and their impact on product quality or shelf life has to be highlighted along with the corrective action.
6.4.4.3 Observe the control sample of the batches under review for their physical appearance and report the same in annual product quality review.
6.4.5 Review of the market complaints.
6.4.6 Review the market compliant register for the batches under review, their investigation report, nature of complaints, reason for occurrence, preventive action to avoid recurrence and record (if any) of training session on the same, and summarize the detail in annual product quality review. Ensure that all the complaints have been investigated and reported.
6.4.7 Review of the Product Recall Reports.
6.4.7.1 Review the product recall report of the selected batches, for the source of complaint, nature of complaint, reason for recall, the distributed quantity, the recalled quantity, reason for discrepancies, the destruction details ( to ensure complete destruction) and investigation report. Compile the data in the annual product quality review along with preventive action to avoid recurrent and their implementation.
6.4.8 Review of Validation Status
6.4.8.1 Review the validation protocols and reports, which have been documented to provide any change in facility, equipment and process during the processing of the batches under review. Record the document number in the annual product quality review.
6.4.8.2 Verify whether the data continues to support validated status. Document the finding, if any discrepancies observed in the validation status during review, recommend the action taken.
6.4.9 Review of Stability Data
6.4.9.1 Review the stability data of finished products. One batch of each finished product, in each type of primary packing must be assessed at the end of the shelf life using appropriate chemical and physical tests.
6.4.9.2 Review the stability data generated within the review period and verify for compliance with the registered or group specifications whichever is tighter.
6.4.9.3 Confirm that the stability data, of the batches under review, supports the shelf life, storage recommendations and overages specified in the MFR.
6.4.10 Review of Export Product (If Export is applicable)
6.4.10.1 For the products manufactured for the export market all the batches manufactured in the year should be reviewed.
6.4.10.2 For new products, where commercially manufactured batches have not reached end of approved shelf life, the stability data from development / validation batches must be reviewed.
6.5 Recommendations
6.5.1 Any discrepancies observed while reviewing should be summarized along with corrective action and the recommendation to avoid the recurrence.
6.6 Retention of Report
6.6.1 The annual product quality review report must be retained for a period of seven years from the date of manufacture of the last batch of product reviewed.
6.6.2 Annual product quality review report will be discussed, and concluded that will be written in report.
6.6.3 Head-Quality Assurance will approve the annual product quality review which will be duly authorized by Chief Executive Officer.
7.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
FG : Finished Goods
RM : Raw Material
PM : Packing Material
MFR : Master Formula Record
APQR : Annual Product Quality Review
8.0 ANNEXURE
| Annexure No. | Title of Annexure |
| Annexure-I | Plan For Annual Product Quality Review |
| Annexure-II | Format for Annual Product Quality Review Report |
ANNUAL PRODUCT QUALITY REVIEW REPORT
GENERIC NAME
PRODUCT NAME
Index
| Sr.No | Table of Contents | Page No. |
| 1. | ||
| 2. | ||
| 3. |
ANNUAL PRODUCT QUALITY REVIEW REPORT OF
INITIATED BY:
| DESIGNATION | NAME | SIGNATURE | DATE |
| EXECUTIVE
(QUALITY ASSURANCE) |
|
REVIEWED BY:
| DESIGNATION | NAME | SIGNATURE | DATE |
| ASST. MANAGER
(QUALITY ASSURANCE) |
|
||
| HEAD
(PRODUCTION) |
|||
| HEAD
(QUALITY CONTROL) |
APPROVED BY:
| DESIGNATION | NAME | SIGNATURE | DATE |
| HEAD
(QUALITY ASSURANCE) |
AUTHORISED BY:
1.0 OBJECTIVE:
2.0 PRODUCT DETAIL:
3.0 REVIEW OF RAW & PACKING MATERIAL QUALITY FINDINGS:
4.0 REVIEW OF RAW MATERIAL (API) DETAILS:
5.0 REVIEW OF CRITICAL PROCESS:
6.0 % YIELD:
7.0 ANYLITICAL RESULTS:
8.0 REVIEW OF FINISHED PRODUCTS QUALITY FINDINGS:
9.0 REVIEW OF ENVIRONMENT MONITORING FINDINGS:
10.0 REVIEW OF DEVIATION:
11.0 REVIEW OF CHANGE CONTROL PROPOSAL:
12.0 REVIEW OF OOS (OUT OF SPECIFICATION):
13.0 REVIEW OF MARKET COMPLAINTS, PRODUCT RECALL:
14.0 REVIEW OF CONTROL SAMPLES :
15.0 REVIEW OF STABILITY MONITORING PROGRAMME :
16.0 REVIEW OF QUALIFICATION STATUS OF EQUIPMENT & UTILITIES:
17.0 CONCLUSION: