sop for approval and release of finished products for market
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure for approval and release of finished product for market.
2.0 SCOPE
2.1 This SOP is applicable for approval and release of finish product for market
3.0 RESPONSIBILITY
3.1 Officer–Quality assurance-Prepare the SOP and follow-up the SOP accordingly
3.2 Asst. manager –Quality assurance -Provide the support to the implementation of SOP and maintained the records.
4.0 ACCOUNTABILITY
4.1 Head – Quality Assurance / Chief Executive Officer
5.0 REFERENCE(S)
5.1 In-House
6.0 PROCEDURE:
6.1 Ensure that the Batch Manufacturing Record has been checked by production and reviewed by QA personnel with respect to all information regarding the various activities of manufacturing and packing and the reconciliation of the materials and product yield are carried at the respective stages of the operation.
6.2 Ensure that Production Head has reviewed the completed BMR before it is submitted to quality assurance for final review and release of the product for sale.
6.3 BMR and BPR shall be reviewed by QA before dispatch
6.4 QA personnel will do terminal inspection in production area after completion of packing and attached the terminal inspection report with BMR / BPR.
6.5 Verify that the product has been released by quality control with respect to it’s testing as per the product release specification. Ensure the Certificate of Analysis (COA) has been signed as token of approval and the entry of A.R. No. & Release date has been made in BMR / BPR and as well as entry in ERP system for batch release.
6.6 After QC approval production department will intimate to QA by ERP system for release of the batch.
6.7 Head QA release the same batch in ERP system and print out of finished goods transfer slip.
6.8 The signature FGT is attached in BMR.
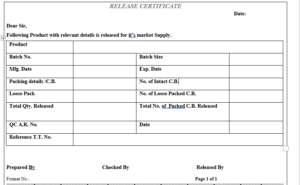
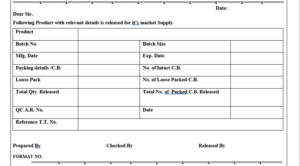
6.9 Prepare “Release Certificate, as per Format No. in duplicate, giving the details of product name, batch details, analytical report number, break up of packed quantity and total quantity released for sale on basis of the batch manufacturing and packing records.
6.10 Verify the quantity being released from quantity transferred to FG Store on ‘Transfer Note’ and as mentioned on the page of the ‘Yield Reconciliation’ in the BMR and on page of the product transfer in BPR.
6.11 Allocate serial number of the release as in record for release notes, sign and submit it along with the batch docket to the Head-QA for final releases of the product for sale and its distribution in market.
6.12 Ensure that the batch manufacturing document is reviewed and batch is released by signing of Head-QA on the specified space of product release in BMR/BPR and on release certificate before product is dispatched from the premise of the plant.
6.13 Send original copy of the “Release Certificate” to FG ware house personnel.
6.14 Enclose the one copy of the release certificate with BMR / BPR. Place the batch manufacturing record in a separate document room.
7.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
BMR : Batch Manufacturing Record
BPR : Batch Packing Record
ERP : Enterprise, Resource & Planning
FG : Finished Goods
FGT : Finished Goods Transfer
8.0 ANNEXURE
| Annexure No. | Title of Annexure |
| Annexure-I | Release Certificate |
ANNEXURE-I