sop for change control procedure
1.0 OBJECTIVE:
The objective of this SOP is:
1.1 To describe the procedure to be followed for experimental change / permanent change.
2.0 RESPONSIBILITY:
2.1 The Originating department shall be:
2.1.1. Responsible for requesting for the Change Control form to Quality Assurance.
2.1.2. Responsible for fill up the Change Control form appropriately.
2.1.3. Responsible for implementation of proposed change after approval of change control.
2.1.4. Responsible for preserving the photocopy of approved Change control after implementation.
2.2 The Executive – Quality Assurance shall be:
2.2.1 Responsible for issuing the photocopy of Change Control form the Master Copy.
2.2.2 Responsible for giving the Change Control Number.
2.2.3 Responsible for preserving the Approved Original Change Control form.
2.2.4 Responsible for maintaining the Change Control Log Book.
2.3 The Head – Engineering shall be:
2.3.1 Responsible for review and recommendation of proposed change, if applicable.
2.4 The Head – Plant Operation (PO) shall be:
2.4.1 Responsible for review and recommendation of the proposed change.
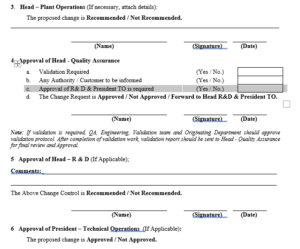
2.5 The Head – R&D shall be:
2.5.1 Responsible for review and recommendation of the change control, if applicable.
2.6 The President – Technical Operations (TO) shall be:
2.6.1 Responsible for review and approval of the proposed change mentioned in change control form, if applicable.
2.7 The Head – Quality Assurance shall be:
2.7.1 Responsible for review and approval of the change control by considering all parameters related to the proposed change.
2.7.2 Responsible for accessing the review and recommendation of Head – R&D and approval of President – Technical Operation, if necessary.
2.7.3 Responsible for verifying the implementation of change by receiving intimation from originating department.
3.0 ACCOUNTABILITY
Head – Quality Assurance
4.0 PROCEDURE
4.1 The change control procedure shall be followed, if any of the following changes are required.
4.1.1 Production
4.1.1.1 Change in Location, equipment, Process control parameters, batch size and Raw Materials / Packaging Materials in Finished Product. Experimental changes planned to generate data prior to any permanent change
4.1.2 Engineering
4.1.2.1 Change in any equipment, critical part of any equipment or Process control parameters in utilities, facility and area layout.
4.1.3 Research & Development
4.1.3.1 Change in Formulation, Specifications or any critical change.
4.1.3.2 Change in source of any raw material or primary packaging materials.
4.1.4 Quality Assurance
4.1.4.1 Change in facility, change in instruments etc.
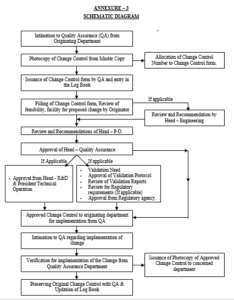
4.2 After receiving the intimation from Originating Department, Executive – Quality Assurance shall take out photocopy of Change control form the Master Copy (Refer – Annexure – 1).
4.3 Allocate a Change Control number to the change control form and issue to the Originating Department. The change control number shall be as CC – 001, for Changes originated by Cephalosporin Production. Whereas CC denotes Change Control and 001 denotes Serial No.
4.4 If any other department is originating the change, then the No. shall be given as CB – 001.
4.5 Update the Change Control Log Book accordingly (Refer – Annexure – 2).
4.6 The originator and Head of Department shall fill the Change Control Form appropriately and review the feasibility, facility for proposed change.
4.7 After filling up, the change control form shall forward to Head – Engineering for review and recommendation, if applicable.
4.8 Then Head – Plant Operation shall review and recommend the change control, if applicable.
4.9 After review and recommendation of Head – Plant Operation, the Change Control form shall forward to Head -Quality Assurance.
4.10 Head – Quality Assurance shall review the request for change based on the necessary details submitted and discuss with Validation team, Head – Plant Operation and Originating Department and decide, whether validation is required or not.
4.11 In case validation is required, then joint decision shall take for following actions,
a. Validation team shall co-ordinate for preparation and approval of protocol and subsequent validation activity.
b. After completion of validation activity the validation report shall be submitted for approval of Head – Production or Head – Engineering or Head Originating Department, Head Plant Operation and Head – Quality Assurance.
4.12 In case no validation is required, then Head Quality Assurance shall make the remarks in the Change Control form and review the other parameters related to the Change Control.
4.13 In case of changes related to product of contract giver approval of contract giver is be taken before implementing the changes.
4.14 If the change control does not require prior approval from regulatory agency then Head – Quality Assurance shall access the necessity of recommendation of Head – R & D and approval by President – Technical Operations.
4.15 If the recommendation of Head R &D and Approval of President – Technical Operation is not required then Head – Quality Assurance shall approve the change control and forward to Executive – Quality Assurance for further actions.
4.16 If the recommendation of Head – R&D and Approval by President – Technical Operation is required then Head Quality Assurance shall make the appropriate remarks in the Change Control Form and forward it to Head – R&D and President – Technical Operations subsequently.
4.17 After the approval by President – Technical Operations, the Original Change Control Form shall be forwarded to Originating Department for implementation through Quality Assurance Department.
4.18 The originating Department on receiving the approved change control form, shall arrange for its implementation.
4.19 The originating Department shall update relevant documents and shall inform the implementation of change to Quality Assurance.
4.20 After getting the intimation from originating Department, Head – Quality Assurance shall verify, implementation of the change and sign on the change control form with remark.
4.21 After that the Original Change Control Form shall be forwarded to Quality Assurance by originating Department.
4.22 Executive – Quality Assurance shall issue the photocopy of approved change control form to the Originating Department for future references.
4.23 The Quality Assurance Department shall retain the Original Approved Change Control form.
4.24 Quality Assurance shall maintain a Log Book of Change Control (Refer Annexure – 2)
5.0 REASON FOR REVISION:
This SOP is modified in order to be more effective in its responsibility and to match the Change Control procedure, designations of authorised persons as per the format and deleted the checklist of change control. The incorporation of Schematic Diagram (Annexure-3) for better understanding of operation and overall review.
6.0 TRAINING:
Trainer — Head – Quality Assurance
Trainees — All Departmental Heads
Period — One day
7.0 DISTRIBUTION:
Certified Copy No. 1 : Head of Department – Engineering
Certified Copy No. 2 : Head of Department – Warehouse
Certified Copy No. 3 : Head of Department – HRD
Certified Copy No. 4 : Head of Department – Information Technology
Certified Copy No. 5 : Head of Department – Materials
Certified Copy No. 6 : Head of Department – Marketing
Certified Copy No. 7 : Head of Department – R & D
Certified Copy No. 8 : Head of Department – Quality Control
Certified Copy No. 9 : Head – Plant Operation
Reference Copy No. 10 : File Copy in Change Control File.
Reference Copy No. 11 : File Copy in Change Control File.
Original Copy : Head – QUALITY ASSURANCE.
8.0 ANNEXURES:
Annexure – 1 : Format for Change Control Form
Annexure – 2 : Format for Change Control Log Book
Annexure – 3 : Schematic Diagram
9.0 REFERENCE:
In-house
ANNEXURE – 1
FORMAT FOR CHANGE CONTROL FORM

ANNEXURE – 1
FORMAT FOR CHANGE CONTROL FORM
ANNEXURE – 2
FORMAT FOR CHANGE CONTROL LOG BOOK
CHANGE CONTROL LOG BOOK