sop for handling of market complaint in pharma
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure for errors and other unpredictable events can result in defective or unsafe
products being released for distribution, with the potential for such goods to cause harm to the people who use them.
This gives rise to complaints. All these complaints must be carefully reviewed and actions must be taken to avoid its recurrence.
In order to provide for all contingencies, a system should be designed to recall, if necessary, promptly and effectively products
known or suspected to be defective, from the market. The objective is to provide a method to be followed in case of any such circumstances.
2.0 SCOPE
2.1 This SOP is applicable for Handling of Market Complaint
3.0 RESPONSIBILITY
3.1 Asst. Officer or above – Quality Assurance: Prepare the SOP and follow up the standard operating procedure accordingly.
3.2 Head – Quality Assurance: Provide the support to the implementation of SOP and maintaining the record.
4.0 ACCOUNTABILITY
4.1 Head – Quality Assurance / Chief Executive Officer
5.0 REFERENCE(S)
5.1 In-House
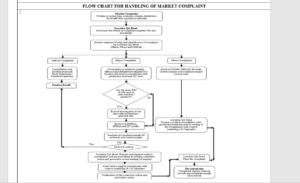
6.0 PROCEDURE
6.1 It is the responsibility of Marketing department to ensure that every complaint about the product is handled on
top most priority respective responsible persons stated as above shall forward all complaints related to products
received from the retailer, wholesaler, patients, physician to Head office or at factory through telephone, courier or email.
He also shall document all the verbal complaints and a report shall be sent to head office or factory within 24 hours of the receipt of the complaint.
6.2 On receipt of the information, the Head of Quality Assurance shall determine whether the defect is
6.2.1 Minor – Unlikely to have an adverse effect on the consumer.
6.2.2 Major – is likely to have an adverse effect on the consumer, but not considered serious, or
6.2.3 Critical – is likely to give rise to a serious adverse effect, or even death, of the consumer.
6.3 Quality assurance has to maintain a register for market complaints as per Format
6.4 Assign a unique, sequential numbering for each market complaint in own products :
MC/YYY/MM/ZZ/NNN
Where:
MC : Market Complaint
/ : For Separation
YYY : Department Code (e.g. PRD: Production)
/ : For Separation
MM : Month in which market complaint occur
/ : For Separation
ZZ : Year in which complaint occur
/ : For Separation
NNN : Serial No. of Market Complaint
e.g.: MC/PRD/MM/ZZ/001…002,.003 etc.
6.5 Assign a unique, sequential numbering for each market complaint in other parties products (If required):
MC/YYY/XX/MM/ZZ/NNN
Where:
MC : Market Complaint
/ : For Separation
YYY : Department Code (e.g. PRD: Production)
XX : Initial of Party Name (e.g.: For Mankind: MK, Emcure: EM etc.)
/ : For Separation
MM : Month in which market complaint occur
/ : For Separation
ZZ : Year in which complaint occur
/ : For Separation
NNN : Serial No. of Market Complaint
e.g.: MC/PRD/MK/MM/ZZ/001…001, MCN/PRD/EM/MM/ZZ/EM/002…. etc.)
6.6 Before sending the complaint detail, inform Chief Executive Officer Whole about the complaint by mail or telephone.
6.7 Forward below mention details, addressed to Head-QA / Chief Executive Officer on the spot when any complaint arises.
• Product Name and Specification
• Batch Number
• Manufacturing Date
• Expiry Date
• Nature of Complaint
• Date of Complaint
• Name, address and sign of person/party/Hospital/Doctor/FDA who has the complaint
• Exact description of the problem
6.8 Document with all the above detail shall send by courier and email along with the product sample, if possible (pack the product sample in such a way that it reaches office/ factory in same condition, as it was packed.) and adverse Drug Event Report to office/factory.
6.9 In case of FDA complaint, they are directly received from Food and Drug control administration or any regulatory body in the form of a typed letter email or telegram.
6.10 Complaint detail shall be received by Head-QA within 24 hours for further investigation. Also the product sample under question shall be sent to Head-QA, if available.
6.11 Head-QA /shall forward this complaint to complainant and copies sent to Chief Executive Officer and concerned Department for “Acknowledgement Letter for Market Complaint” as per Format along with the Certificate of analysis (COA), if required, of the batch in question to originator on the same day of receipt. The copy of the same shall be sent to Chief Executive Officer simultaneously investigation shall be started by Head-QA / Executive.
6.12 The complaint shall be recorded in the complaint register.
6.13 Head Quality Assurance is designated as responsible for handling market complaints and deciding the measures to be taken.
6.14 Head-QA shall investigate first regarding the authenticity of the complaint. If after investigation it is found that it was just the mistake or misconception of the complainer, he shall document the same and close the complaint. He shall send the copies of the same to the Chief Executive Officer.
6.15 In case, if the complaint is found to be genuine, check if it is for goods shortage or a quality related compliant. Incase of the goods shortage investigate the cause, alert the line crew and send the reply to the Chief Executive Officer , complainer and close the complaint.
6.16 If the complaint is related to the quality, check all the parameters on the available product sample or the retain sample of the same batch. (With prior permission of Chief Executive Officer) if required.
6.17 Carry out assessment of any abnormal parameters, observed on the sample compared to the retain sample. If required perform analysis.
6.18 Then after, Head-QA, Head-QC and Head-Production shall jointly analyze the findings observed during the investigation with max. Possible ways. They can review a batch job card for this purpose.
6.19 If after the findings any change is to be required either in the process or in the Equipment shall be done prior to the permission of the Chief Executive Officer and to be done according to the process of change control. This whole process shall be documented in the investigation report.
6.20 The investigation report shall include at least the observations on the following as per Format
Complaint Sample
Control Sample (Retain Sample)
Reference Standard (if required)
Batch Manufacturing Records
Related Records for Batch Manufacturing
Past History of the Product
Ongoing Stability Studies Status
Possible Causes
Conclusion
Customer / Party Approval (Required / Not Required)
Corrective action taken (Jointly discussed with Chief Executive Officer).
Preventive action taken (Jointly discussed with Chief Executive Officer).
6.21 In case of the complaint from FDA or any regulatory body, a certificate of analysis, retesting results along with 3 sets of photocopy
of the batch job card of that batch, in question is submitted to the regulatory, if required.
6.22 In case of the complaints other than sterility failure all the reports are submitted within a max. of 7-8 days & in case of sterility
failure within a max. of 8-16 days of the receipt of the complaint to the factory.
6.23 If upon investigation it appears that the batch was released by inadvertent chance mistake or there is some discrepancy in the result,
due to which it is felt that it should be subjected to retest, Then the marketing people is informed in written to obtain the samples of the
batch in question from the nearest source. If fails in getting samples Head-QA may perform re analysis using retain samples.
6.24 If a product defect is discovered or suspected in a batch, consideration should be given to checking of other batches in order to determine
whether they are also affected. In particular if there is any other batch, which contain Operation of the defective batch should be investigated.
6.25 Upon reanalysis if the batch passes, marketing dept. is informed with the necessary records.
6.26 If, on reanalysis the batch fails, Head-QA, Head-QC shall recommend the Chief Executive Officer for recalling the batch from
the market with immediate effect. After the authorization from the Vice President-Operations, Head-QA shall ask the concerned
marketing departmental head to send back total quantity to the factory for further action.
6.27 The FDA authorities shall be informed if a action is considered following possibility faulty manufacture, product
deterioration, or any other serious quality problem with a product.
6.28 Investigation report should be informed to:
• Originator of the Complaint
• The concerned Departments
• The Customer / Party (Which has related with Product)
6.29 After sending the investigation report if no feedback is received from customer / complainer within one month of sending the investigation
report, the market complaints should be closed. In case of critical market complaint do not close the complaint till investigation is not completed.
6.30 Finally a complaint closure letter should be sent to the originator as per Format
6.31 Complaint records should be reviewed yearly for any indication of specific or recurring problem requiring attention and possibly recall from the market.
8.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
QC : Quality Control
FDA : Food & Drug Administration
9.0 ANNEXURE
| Annexure No. | Title of Annexure |
| Annexure-I | Flow Chart for Handling of Market Complaint |
| Annexure-II | Acknowledgment Letter for Market Complaint |
| Annexure-III | Investigation Report for Market Complaint |
| Annexure-IV | Complaint Closure for Market Complaint |
| Annexure-V | Market Complaint Log Book |
ANNEXURE-I
ANNEXURE-II

ANNEXURE-III
INVESTIGATON REPORT FOR MARKET COMPLAINT
| Complaint Number | |||||||||
| Complaint Received From | Date: | ||||||||
| Complaint Sample Received Mark (✔) | Yes No | ||||||||
| PRODUCT NAME & DETAILS | |||||||||
| Product | Composition | Batch
No.
|
Mfg. Date | Exp. Date | Pack
|
Recommended
Storage Conditions |
|||
| Nature of Complaint |
|
||||||||
| Classification of Complaint Mark (✔) | Minor Major Critical | ||||||||
| INVESTIGATION DETAILS | |||||||||
| Observations On Complaint Sample | |||||||||
| Observations On Control Sample |
|
||||||||
| Observations On Batch Manufacturing Records |
|
||||||||
| Observations on Production & Packing Logbooks |
|
||||||||
| Observations on Quality Control Data: |
|
||||||||
| Note: Following Guide Line shall be followed: | |||||||||
| 1. Human Error: List out identified personnel involved | |||||||||
| 2. Machine Related: Check engineering / machine log books for any break down failure. Check adequacy of safety systems and controls | |||||||||
| 3. Process Related: Checks for any deviations/ discrepancies / non-compliance. | |||||||||
| 4. Material Related: List of AR numbers source & check initial test reports. |
|
||||||||
| Summary & Conclusion |
|
||||||||
| Party / Customer Approval
(Required / Not Required) |
Party / Customer Sign / Date
|
||||||||
| Corrective Action Taken |
|
||||||||
|
Preventive action (Planned) |
|
||||||||
ANNEXURE-IV
COMPLAINT CLOSURE FOR MARKET COMPLAINT
| Complaint Number | |||||||
| Complaint Opening Date | |||||||
| PRODUCT NAME & DETAILS | |||||||
| Product | Composition | Recommended
Storage Conditions |
Batch No. | Manufacturing Date | Expiry Date | ||
| Complaint received from | |||||||
| Comments of complainant (Originator) | |||||||
| Mode of communication | |||||||
| Whether the Samples given by complainant?
If yes, when? |
|||||||
| Acknowledgment sent on | |||||||
| Acknowledgment sent to | |||||||
| Acknowledgment copies sent to | |||||||
| Investigation Report Sent On: | |||||||
| Summary of Complaint Closure:
|
|||||||
|
Dated: Head – QA |
|||||||
| CC: Chief Executive Officer, Concerned Department HOD and Originator of complaint | |||||||
