sop for handling of non conformance incidence
1.0. OBJECTIVE:
The objective of this SOP is:
1.1 This procedure defines the responsibilities and authority for handling and investigating non-conformance (NC), for taking appropriate corrective and preventive actions to prevent recurrence of the same.
2.0. RESPONSIBILITY:
2.1 Any employee of the organization shall be:
2.1.1 Responsible for originating Non-conformance report on observation of any Non-conformance in factory premises and forward to Head / Section in-charge of concerned department.
2.2 Head / Section in-charge of the Concerned Department / In-Process QA Personnel shall be:
2.2.1 Responsible for taking necessary corrective and preventive actions and forward to Quality Assurance to approval of the same.
2.2.2 Responsible for implementation of corrective and preventive actions.
2.3 Quality Assurance shall be:
2.3.2 Responsible for reviewing and approving the report and their respective corrective and preventive actions.
2.3.3 Responsible for ensuring the closing of the non-conformance in co-ordination with the responsible person of concerned department.
2.3.4 Responsible for the periodically review the non-conformance report.
2.3.5 Responsible for registration of the NCR after the approval of Head – Quality Assurance and maintaining the logbook of NCR.
3.0. ACCOUNTABILITY:
Head – Quality Assurance
4.0. DEFINITIONS AND PROCEDURE:
4.1 Categorization of NC:
4.1.1 Minor: Deviation for standard operating procedure or cGMP violation, which cannot lead to what is described under Major and Critical.
4.1.2 Major: Any deviation from the validated / established procedure, process, system and practices.
4.1.3 Critical:
4.1.3.1 Critical NC is any NC, which can affect the purity, strength and safety of the medicine, which may pose serious health risk to the user.
4.1.3.2 Any minor deviation, which has been repeated 10 times shall be, consider as a critical.
4.1.3.3 Repetition of any major NCR more than 5 times shall be consider as a critical.
4.2 Corrective & Preventive Actions:
4.2.1 Corrective Action: Action taken to rectify the non-conformance.
4.2.2 Preventive Action: Action taken to avoid the re-occurrence of the same non-conformance. This can involve modification or enforcement of procedures or implementation of further controls.
4.3 Identification of Non-Conformance:
4.3.1 Any one can raise non-conformance, who found any deviation in the factory premises.
4.3.2 The non-conformance should be very specific with respect to Name of the Department, area, person, Date, reference of SOP/System/Practice / Product / Batch No. / Manufacturing Stage, where relevant.
4.3.3 If the non-conformance is of major / critical category a copy of the same shall be given to unit head for his information and necessary actions. If it is of critical category, it should be approved and closed by Head – Technical Operation.
4.4 Probable cause:
4.4.1 Describe possible cause(s) / reason of the non-conformance.
4.4.2 By whichever means of a non-conformance is identified, the underlying cause(s) of the non-conformance must be investigated.
4.5 Corrective Action:
4.5.1 Appropriate and timely corrective action must be proposed according to the nature of the non-conformance by concerned department and it shall be implemented only after the Approval of Quality Assurance.
4.5.2 If required, advice can be taken from Quality Assurance.
4.6 Preventive Action:
4.6.1 Preventive action such as implementing modifying or enforcing procedures or controls shall be taken to avoid recurrence of the non-conformance.
4.6.2 The proposed action can be proposed by concerned department and shall be implemented only after approval of Quality Assurance.
4.6.3 If required, advice can be taken from Quality Assurance.
4.6.4 The Non-conformance report shall be submitted to the Head – Quality Assurance. The report shall include the following:
• Categorization of Non-conformance
• Description of the non-conformance observed
• Probable cause(s) for the Non-conformance
• Proposed Corrective actions
• Proposed Preventive actions
4.6.5 The Head – Quality Assurance shall review the Non-Conformance report for its magnitude, if found required, Head – QA shall forward it to Head – Technical Operations.
4.6.6 On the basis of review of Head – Quality Assurance, he shall approve / Reject the corrective action / preventive actions. If Head – Quality Assurance rejects the corrective / Preventive action taken, he shall recommend the necessary corrective / Preventive actions.
4.6.7 While closing the non-conformance report, the Quality Assurance person shall verify that the proper corrective / preventive actions have been taken.
4.7 Documentation:
4.7.1 Each non-conformance report shall be raised in the format given in Annexure – 1, by the originator.
4.7.2 Each Non-conformance incidence report shall be registered in Quality Assurance and QA gives a Registration number and maintain the same in Logbook after the approval of Head – Quality Assurance. If required, advice can be taken from Quality Assurance.
4.7.3 The number is comprised of 8 characters.
4.7.4 The first character is an alphabet followed by the “/” character, which represents the responsible Department for non-conformance as listed below:
Production : G
Engineering : E
Ware house : W
Personnel & Administration : P
Quality Assurance / Quality Control : Q
4.7.5 The next 2 Characters represents the year in which the non-conformance is registered, followed by “/”.
4.7.6 The next 3 Characters represents the Sr. No. starting from 001.
For example “G/03/002”
In the above registration number the Character “G” represents Production General “03” states the year 2003 and “002” represents the second Non-conformance registered in the year.
4.7.7 Non-conformance corrective action / Preventive actions shall be reviewed half yearly by Head – Quality Assurance and Head- Plant Operations to review any specific trend of the same and reconsider the Corrective and Preventive Actions taken, if required.
4.7.8 Original copy of the Non-Conformance report retained by Quality Assurance Department and one photocopy shall be issued to concerned department.
4.7.9 If the Non-conformance is related to production batch(s), a photocopy of the same shall be made a part of Batch Manufacturing Record, so as to enable Quality Assurance to consider the impact of the same before releasing the batch.
5.0. REASON FOR REVISION:
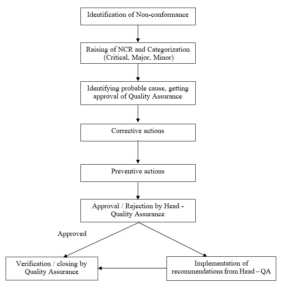
The SOP is revised to incorporate the identification procedure, preventive action and periodic review of Non-Conformance reports, by Head – Plant Operation and Head Quality Assurance. The schematic diagram is also incorporated for better understanding of the procedure.
6.0. TRAINING:
Trainer — Head – Quality Assurance
Trainee — All Departmental Heads / Quality Assurance Personnel
Period — One day
7.0. DISTRIBUTION:
Certified Copy No. 1 : Head of Department – Quality Control
Certified Copy No. 2 : Head of Department – General
Certified Copy No. 3 : Head of Department – Warehouse
Certified Copy No. 4 : Head of Department – Engineering
Certified Copy No. 5 : Head of Department – Personnel and Administration
Certified Copy No. 6 : IPQA
Certified Copy No. 7 : Head – Plant Operation
Original Copy : Head – QUALITY ASSURANCE
8.0. ANNEXURE:
Annexure – 1 : Format for Non-Conformance Report.
Annexure – 2 : Format for Recording the Summary of Non-Conformance Reports.
Annexure – 3 : Format For Log Book Of Non-Conformance Reports
Annexure – 4 : Schematic Diagram
9.0. REFERENCE:
Quality System Inspection and In-house
ANNEXURE – 1
FORMAT FOR NON-CONFORMANCE REPORT
| IDENTIFICATION | |||
| Department: | Area: | NC No.: | |
| Category: Minor / Major / Critical | C.C.: | ||
| Description of Non Conformance*:
Name: Sign./Date:———————– |
PROBABLE CAUSE |
|
Concern Dept.: Quality Assurance: |
CORRECTIVE ACTION |
|
Description*:
|
Responsibility: —————- |
| TCD : ————————– | |
| Sign./Date:——————— | |
PREVENTIVE ACTION |
|
Description*: |
Responsibility: —————- |
| TCD : ————————– | |
| Sign./Date:——————— | |
QUALITY ASSURANCE COMMENT * |
|
Sign./Date:———————– |
CLOSING OF THE NON -CONFORMANCE |
|
Sign./Date:———————– |
ANNEXURE – 2
FORMAT FOR RECORDING THE SUMMARY OF NON-CONFORMANCE REPORTS
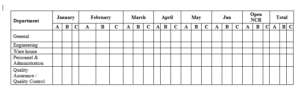
A- Minor, B- Major and C-Critical
ANNEXURE – 3
FORMAT FOR LOG BOOK OF NON-CONFORMANCE REPORTS
| Sr. No. | Date | Originator | Department / Area | Description | NC No. | NCR Closed On | Closed by |
ANNEXURE – 4
SCHEMATIC DIAGRAM
Thanks
i have learned and be able to have an idea of NCR how doe it look like