sop for mock recall in pharma
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure of mock recall to evaluate the effectiveness of the arrangements of recall procedure.
2.0 SCOPE
2.1 This SOP is applicable to finished products to mock recall which were manufactured
2.1 Procedure for mock recall for domestic market is applicable to receiving reply of mock recall intimation from distributors (C&F), stockiest and marketing managers (physician sample/clinical test samples). And for export market it is applicable to receiving reply of mock recall intimation from distributors.
3.0 RESPONSIBILITY
3.1 Asst. Manager /Executive QA shall be responsible for initiation of mock recall to Distribution Head and Marketing Head (domestic and/or export division).
3.2 Distribution Head and Marketing Head are responsible to provide the stock statement from all stake holders.
4.0 ACCOUNTABILITY
4.1 Head – Quality Assurance Department / Chief Executive Officer
5.0 REFERENCE(S)
5.1 In-House
6.0 PROCEDURE:
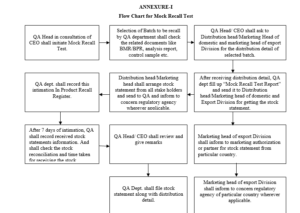
6.1 If no actual product recall is made in a year, Asst. Manager QA/Head QA shall initiate the mock recall test in the month of December as per below mentioned procedure.
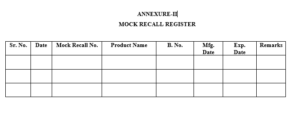
6.2 QA personnel shall allocate mock recall no. as ‘MR/YY/ZZ where, MR stand for Mock Recall and YY last two digits of year, ZZ stand for serial no..Officer/Executive QA shall record mock recall in ‘Mock Recall Register’ as per Format
6.3 QA Executive/Asst. Manager shall select any batch of any finished product (from BPR) which was dispatched to most of the following stake holders:
6.3.1 Domestic supply
6.3.2 Export supply
6.3.3 Physician / Clinical test samples
6.4 After selection of product and batch, QA Head Coordinate with Chief Executive Officer, ask the distribution detail of this batch from Distribution Head (if supplied for domestic) , Marketing Head of domestic division (if supplied as physician / clinical test samples) and/or Marketing Head of export division (if supplied for export).
6.5 After receiving the distribution detail, QA Head or his/her designee shall fill up Part-I of ‘Mock Recall Test Report’ as per Format which seek the stock statement of all stake holders and send this annexure to Distribution Head (if supplied for domestic), Marketing Head of domestic division (if supplied as physician / clinical test samples) and if supplied export, then the Marketing Head of export division shall intimate to the marketing authorization partner for each individual country wherever distributed who shall make necessary arrangement for mock recall of product from the particular country with intimation to concern regulatory agency whenever applicable.
6.6 Distribution Head and/or Marketing Head (domestic and/or export) shall arrange stock statement from all stake holders as mentioned in Part-I of Format
6.6.1 QA Executive/Asst. Manager shall record this intimation in Mock Recall Register.
6.6.2 After seven days of intimation, shall record the received stock statements information in Part-II of Format
6.6.3 After receiving stock statements from all stake holders, QA Executive/Asst. Manager shall fill up rest of the information in Part-II of Format and hand over the same to QA Head for his review.
6.6.4 QA Head shall review the report and give his remarks and acknowledge the same.
6.6.5 QA Officer/Executive shall file filled Format and all stock statements (stake holder-wise) along with distribution detail.
6.6.6 QA Officer/Executive shall check the BMR/BPR, Stores Copy, QC Report & control sample of Mock Recall Batch.
6.6.7 Attach the Photocopy of BMR/ BPR, Invoice Copy, Security out and Email copy with the Mock Recall Report.
6.7 Documentation
6.7.1 Flow Chart for Mock Recall as per Format
6.7.2 Mock Recall Register as per Format
6.7.3 Mock Recall Test Report for Product Recall as per Format
7.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
Mfg. : Manufacturing Date
Exp. : Expiry Date
C&F : Carrying and Forwarding
ZBM : Zonal Business Manager
RBM : Regional Business Manager
BMR : Batch Manufacturing Record
BPR : Batch Packing Record
8.0 ANNEXURE
| Annexure No. | Title of Annexure |
| Annexure-I | Flow Chart for Mock Recall Test |
| Annexure-II | Mock Recall Register |
| Annexure-III | Mock Recall Test Report for Product Recall |

good