sop for status labeling of equipment and product
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure for define the procedure for status labeling procedure for equipment.
2.0 SCOPE
2.1 This SOP is applicable for Status Labeling Procedure of Equipment
3.0 RESPONSIBILITY
3.1 Executive/Officer –Concerned Department/Quality Assurance
4.0 ACCOUNTABILITY
4.1 Head – Concerned Department/Quality Assurance Department
5.0 REFERENCE(S)
5.1 In-House
6.0 PROCEDURE
6.1 Each Machine / Equipment has its Identification Tag / Label.
6.2 Each Machine / Equipment has Tag / Label indicating the status of cleanliness such as Cleaned / to be cleaned
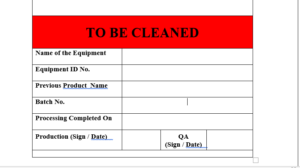
6.3 After use of equipment the label will Be affixed on the equipment “TO BE CLEANED”..
6.4 The specimen copy of the label “TO BE CLEANED” attached as Format
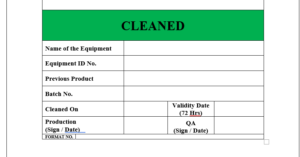
6.5 After cleaning of equipment the label will be affixed on the each machine /equipment is “CLEANED”.
6.6 The label “CLEANED” is to be affixed on the clean equipment which indicates date of cleaning and signature of officer mentioning its validity period.
6.7 The specimen copy of the label “CLEANED” attached as Format
6.8 If any equipment /machine out of order the label will be affixed on the equipment / machine “UNDER MAITENANCE”.
6.9 The label “UNDER MAITENANCE” is to be affixed on the equipment which indicates that the machine is out of order or under maintenance.
6.10 The specimen copy of the label “UNDER MAITENANCE” attached as Format
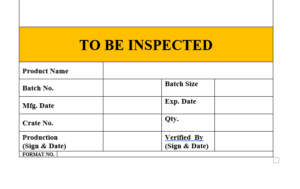
6.11 After completion of filling of product ready for sterilization after sterilization label will be affixed on the product “TO BE INSPECTED”.
6.12 The label “TO BE INSPECTED” is to be affixed on the products, which indicate that the product is not inspected till now.
6.13 The specimen copy of the label “TO BE INSPECTED” attached as Format
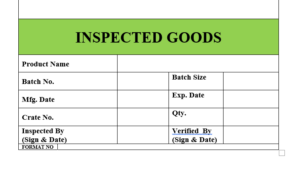
6.14 After completion of visual inspection the label will be affixed on the product “INSPECTED”.
6.15 The label “INSPECTED” is to be affixed on the products which indicate that the product is inspected by visual inspector.
6.16 The specimen copy of the label “INSPECTED” attached as Format No.
6.17 Any stage of manufacturing and packing of product the label will be affixed on the rejected material “REJECTED”
6.18 The label “REJECTED” is to be affixed on the products which indicate that the product is rejected during manufacturing or packing operation as per Format No.
6.19 The specimen copy of the label “REJECTED” attached as
6.20 After labeling all the label vials / ampoules the label will be affixed on the labeled vials and ampoules “LABELLED”
6.21 The label “LABELLED” is to be affixed on the products which indicate that the product is labeled and ready for final packing in carton or shirnk.
6.22 The specimen copy of the label “LABELLED” attached as Format No.
6.23 Affixed the “STATUS LABEL on crate / equipment / area whether any activity is going on or not.
6.24 The label “STATUS LABEL” is to be affixed on the equipment / area which indicate status of area / equipment
6.25 The specimen copy of the label “STATUS LABEL” attached as Format No. and If any batch hold in any stage due to some problem and any deficiency affixed the label on each shipper “HOLD SLIP”.
6.26 The label “HOLD SLIP” is to be affixed on the product / machine / area which indicate the product is under hold.
6.27 The specimen copy of the label “HOLD SLIP” attached as Format No.
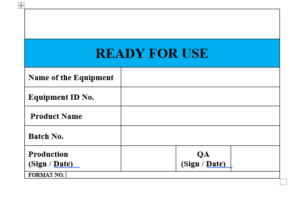
6.28 Affixed the label “READY FOR USE” before using of cleaned equipment
6.29 The label “READY FOR USE” is to be affixed on the machine / area which indicate the equipment / area is ready for use after cleaning.
6.30 The specimen copy of the label “READY FOR USE” attached as Format No.
6.31 If any material goes for QC testing affix the label “UNDER TEST” as per the Format No.
7.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
8.0 ANNEXURE
| Serial No. | Title of Annexure |
| 1 | To Be Cleaned |
| 2 | Cleaned |
| 3 | QA Release |
| 4 | To be Inspected |
| 5 | Inspected Goods |
| 6 | Rejected |
| 7 | Labeled |
| 8 | Status Label |
| 9 | Hold Slip |
| 10 | Ready For Use |
| 11 | Under Test |
ANNEXURE I
ANNEXURE II
ANNEXURE III
| QA RELEASE
A.R. No.: Sign / Date Format No. : |
ANNEXURE IV
ANNEXURE V
ANNEXURE VI
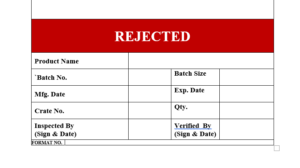
ANNEXURE VII
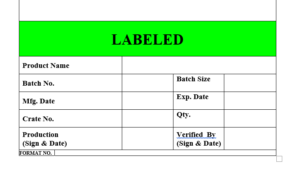
ANNEXURE VIII
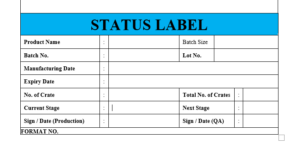
ANNEXURE X
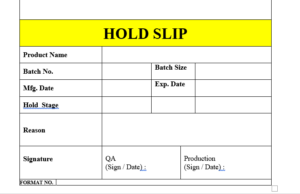
ANNEXURE XI
ANNEXURE XII
| UNDER TEST
Sign/ Date (QA): Format No.: |