Calibration & Verification Of Analytical Balance
1.0 Objective
To lay down a guidelines for Calibration & Verification of Analytical Balance in Quality Control Department.
2.0 Scope
This SOP applies to Calibration of Analytical Balance used in Quality control Lab in abc company
3.0 Responsibility
3.1 Executive – Quality Control.
3.2 In charge/Head.
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
QC : Quality Control
CC NO : Change Control Number
cGMP : Current Good Manufacturing Practice
C : Calibration
SD : Standard deviation
gm. : Gram
5.0 Procedure
5.1 Handling of Weight Box
5.1.1 Store the weight box in dust free area.
5.1.2 Do not touch the weight with bare hand, for handing the weight use a forceps.
5.1.3 Use certified weights only.
5.1.4 For cleaning of weights use a lint free cloth moistened with small amount of Di-ethyl ether and air dry.
5.1.5 Do not drop the weight on the pan to avoid any damage to the balance. Gently place the weight on the center
of the pan to eliminate comer weighing differences.
5.2 General Instruction
5.2.1 Clean the pan and Plat form of the balance after weighing is over.
5.2.2 Do not over load the balance of their maximum capacity.
5.2.3 Keep doors of the balance always closed.
5.2.4 Before closing and opening, ensure that the pan is empty.
5.2.5 Keep the balance in “STAND BY” position when not in use, by pressing I/Q’ key of the balance.
5.3 Cleaning
5.3.1 Before cleaning, disconnect the balance from the power supply.
5.3.2 Do not use any aggressive cleaning agents or strong solvents.
5.3.3 Use a piece of cloth which has been wet with a mild detergent (soap) to clean the weighing pan and balance housing.
5.3.4 Make sure that no liquid enters the balance housing.
5.3.5 After cleaning, wipe down the balance with a soft, dry piece of cloth.
5.3.6 Carefully remove any sample residue/ spilled powder by using a brush that is placed with the balance.
5.4 Daily verification of Analytical Balance
5.4.1 Internal calibration (Self-calibration/ Auto calibration)
5.4.1.1 Press “CAL” key. Balance will go into automatic self calibration mode and “CAL” will be displayed,
the “CC” will be displayed after self calibration is over display will show 0.000 g, then balance is
ready for use. Record the observation in the given Annexure-1.
5.4.2 With Secondary standard weight
5.4.2.1 Calibration the balance daily with the standard weights of 1 g, 10g, 100 g & 200 g individually. Record
the results as given in Annexure-1.
5.4.2.2 Acceptance Criteria: ±0.1% of the Certified weight.
5.5 Monthly Calibration od Analytical Balance
5.5.1 Measurement of Uncertainty
5.5.1.1 Ensure that the standard weights used are within the validity period of its calibration.
5.5.1.2 The weights selected for uncertainty measurement are 1 g, 5 g & 10 g.
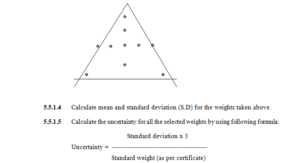
5.5.1.3 Measure the weights by keeping the standard weight at the positions (10 times, individually)
as given in the following diagram.
5.5.1.6 Acceptance criteria for uncertainty measurement: NMT-0.001
5.5.1.7 Record the observations in annexure-2.
5.5.2 Calibration with standard weights
5.5.2.1 Place 1 g certified weight in the center of the pan. Close the door.
5.5.2.2 Wait until the weighing result is stable.
5.5.2.3 Now read the displayed weight.
5.5.2.4 Take out the 1 g weight and allow the balance to come to zero.
5.5.2.5 Repeat the above procedure for the certified weights of 2g, 5g, 10g, 20g, 50g, 100g and 200g.
5.5.2.6 Record the observations in annexure-2.
5.5.2.7 Acceptance Criteria: ±0.1% of the certified weight.
6.0 Forms and Records
6.1 Daily verification record of Analytical Balance – Annexure-1
6.2 Monthly calibration of Analytical Balance – Annexure-2
7.0 Distribution
7.1 Master copy – Documentation Cell (Quality Assurance)
7.2 Control copy – Quality Control
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |
Operating Procedure of Centrifuge
sop for preparation and storage of reagent solution
sop for Calibration and Maintenance of Laboratory Instruments and Equipment
Disposal of Residual Sample or Left Over Material
sop for for Laboratory Incident
standard operating procedure temperature monitoring
sop for operation of infrared moisture balance
sop for preparation of mobile phase
sop for Preparation and Issuance of Analysis protocol standard
sop of placebo and impurity stock solutions
sop for disposal of residual sample
sop for handling of pharmacopoeial changes
sop for procedure for operation of ultrasonic cleaner
difference between UPLC and HPLC
sop for for Emergency Eyewash and Shower
sop for operation and calibration of total organic carbon analyzers
sop for operation of cobb tester
sop for Operation and calibration of atomic absorption spectrophotometer
sop for Operation and calibration of gas liquid chromatograph
sop for operation of humidity oven
sop for operation and calibration of serological water bath
sop for monitoring of drain trap
sop for destruction of analytical samples after testing and control samples
sop for destruction of used chemicals
Sop for Operation of suction pump
sop for Operation and calibration uv cabinet
sop for Operation and calibration of bulk density apparatus
sop for operation and calibration of shore hardness tester
sop for operation of rub proofness tester
sop for monitoring of purified water
sop for Retesting of packaging materials
sop for Retesting and resampling of raw materials
sop for Control of issuance of record of analysis green sheets
sop for Control of computer passwords
sop for sampling of packaging materials PM