sop for handling of deviations
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure for handling of all deviations observed at any stage either during receipt and handling of RM /PM or during it’s storage, during processing, testing, manufacturing and packaging operations involved during manufacture of pharmaceutical finished products.
2.0 SCOPE
2.1 This SOP is applicable for handling of all deviations observed at any stage either during receipt and handling of RM / PM or during it’s storage, during processing, testing, manufacturing and packaging operations involved during manufacture of pharmaceutical finished product’s formulated
3.0 RESPONSIBILITY
3.1 Asst. Officer or above –Quality assurance – Prepare the SOP and follow-up the SOP accordingly
3.2 Asst. Manager – Quality Assurance-Provide the support to the implementation of SOP and maintained the records.
4.0 ACCOUNTABILITY
4.1 Head – Quality Assurance / Chief Executive Officer
5.0 REFERENCE(S)
5.1 In-House
6.0 PROCEDURE
6.1 Definition
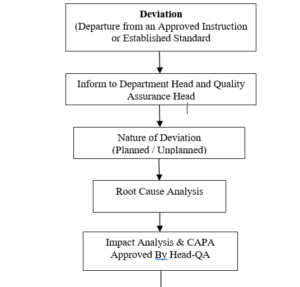
6.1.1 Deviation: Any planned or unplanned disappearance from an approved instruction or established standard is a deviation.
6.1.2 Planned Deviation: A planned departure is a proposed change to any approved procedure, document or specification prior to execution.
6.1.3 Unplanned Deviation: An unplanned departure is an unexpected event that requires a change to any approved procedure, document or specification. It is usually discovered after the fact.
6.1.4 Major Deviation (equivalent to critical deviations): Any planned / unplanned deviation from any established standard which may have any impact upon the identity, Quality, purity, stability, safety, physical characteristics, polymorphism and efficacy of the product is a major deviation.
6.1.5 Minor Deviation: Any planned / unplanned deviation from any established standard which may not have any impact upon the identity, Quality, purity, stability, safety, physical characteristics, polymorphism and efficacy of the product is a minor deviation.
6.1.6 Any deviation (planned / unplanned) must be reported immediately to the Head-concerned department.
6.1.7 Head-concerned department inform about the deviation to the Head-QA & request for the deviation form “Deviation / Remark Report” as per Format
6.2 QA department issue the deviation form after allotting deviation number to the concerned department.
6.3 The deviation number should be given as DVN/YYY/MM/ZZ/NNN:
Where:
DVN : Deviation
YYY : Department Code (PRD= Production, QCD=Quality Control
WHD= Ware House Department, ENG= Engineering
MM : Stand for month in which deviation occur
ZZ : The year of finding the deviation in two digits.
NNN : Serial number of deviation in three digits
(E.g. DVN/PRD/01/15/001, DVN/QCD/01/15/001, DVN/WHD/01/15/001)
6.4 The same must be entered on “Deviation Log” as per Format
6.5 Concerned department fill the deviation particulars in deviation form at specified place.
6.6 QA establish the nature of deviation i.e. major or minor.
6.7 Concerned department also write the immediate action which have been taken on the deviation form.
6.8 QA & concerned department along with the person involved analyzed the root cause of deviation.
6.9 On the basis of root cause analysis, QA suggest a proposed corrective action keeping in mind the post impact of particular action.
6.10 For more suggestions or right actions for proposed corrective action & its impact, deviation form may be referred to any other related department / authority, if it is required.
6.11 Concerned department take the proposed corrective action under the supervision of Head-concerned department.
6.12 After successful accomplishment of corrective action, QA proposed the preventive action considering root cause analysis & corrective action so that the re-occurrence of the deviation can not be repeated. Proposed preventive action may contain re-validation of process / equipment or re-training of personnel.
6.13 Concerned department take the proposed preventive action under the supervision of Head- concerned department.
6.14 After corrective & preventive action taken, Head-QA along with Head-concerned department & Chief Executive Officer verify the effectiveness of action taken and Head-QA give the comments, conclusion over the handling of deviation and approved the same.
6.15 After approval of QA, deviation shall be authorized by Chief Executive Officer with sign & date.
6.16 Deviation approval is a specific one time use document that modifies the documents referenced in the related BMR/BPR, SOP, specification & others. It does not change permanently existing documents.
6.17 Based on the investigation, a permanent change can be implemented as per the SOP for Change Control, in case of major deviations.
6.18 If the minor deviation occurs frequently, then, a trend is to be made for those deviations. Based on the trend obtained, a permanent change can be implemented.
6.19 A review of all major deviation should be done during the Annual product review.
6.20 If any planned and unplanned deviation occurs file up the deviation in the deviation log within same day.
6.21 In case of minor deviation, the target period of closing of deviation is same day.
6.22 In case of major deviation, the target period of closing of deviation is 30 days.
6.23 If the deviation form does not close within its time period, an explanation with reason is required and pending deviation report forward to the higher management for necessary action.
7.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
BPR : Batch Processing Record
BMR : Batch Manufacturing Record
ANNEXURE