sop for handling of incidents
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure for handling of incidents.
2.0 SCOPE
2.1 This SOP is applicable for handling of incident at production area
3.0 RESPONSIBILITY
3.1 Asst. Officer or Above –Quality Assurance – Prepare the SOP and follow-up the SOP accordingly.
3.2 Asst. Manager –Quality Assurance -Provide the support to the implementation of SOP and maintained the records.
4.0 ACCOUNTABILITY
4.1 Head – Quality Assurance Department / Chief Executive Officer.
5.0 REFERENCE(S)
5.1 Not Applicable
6.0 ENVIRONMENT, HEALTH AND SAFETY
6.1 Not Applicable
7.0 PROCEDURE
7.1 On occurrence of incident the employee shall immediately inform, verbally or telephonically to the concerned supervisor /shift-in-charge, who shall decide whether to continue the process or stop the process.
7.2 Supervisor /Shift-in-charge shall inform the concerned head of department or his/her designee.
7.3 Incident shall be immediately reported by the concerned department head to quality assurance department and Chief Executive Officer.
7.4 QA shall issue incident report form to concerned department.
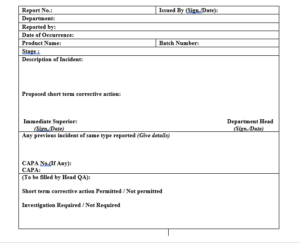
7.5 Any person can report the incident through the incident report as per Format No. CLS/QAD/015/F01 counter signed by immediate superior and department head of the reporter.
7.6 Concerned department shall fill the details as mentioned in the form and submit to QA.
7.7 QA shall assign incident report number to the form. Incident report number shall comprise of following characters:
IR/YYY/MM/ZZ/001
Where:
IR = Incident Report
/ = Separation
YYY = Department Code (PRD = Production Department)
MM = Month
/ = Separation
ZZ = Denotes the calendar year in which incident occur.
NNN = Serial Number like 001, 002…….etc.
7.8 Incident number shall change every year.
7.9 Executive/Officer QA shall be submit the form to Head-QA for review.
7.10 Head-QA shall review the form and as per requirement permit designated persons from QA and concerned department’s to investigate the incident.
7.11 If any CAPA need to fill the undertaken same shall be done as per SOP
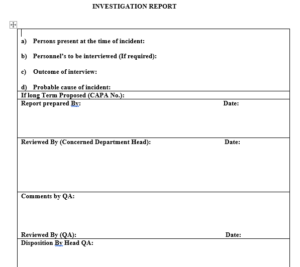
7.12 The designated person from concerned department shall submit completed investigation report as per Format to his/her department Head for review.
7.13 Concerned Department Head shall approve the report and report shall be submitted to QA.
7.14 QA personnel shall review the incident and conclude the cause of incident in his/her comments.
7.15 If required corrective action shall be planned and CAPA shall be filled.
7.16 Finally completed report shall be submitted to Head-QA for disposition.
7.17 After implementation of proposed corrective action Incident report shall be closed by Executive / Officer-QA.
7.18 Authorization Note:
7.18.1 For incidents (change in any process or procedure, exhaustion of inventory, Use of different specification at different locations, etc.) which do not have any effect on product quality, purity and strength under the recommendation of QA in consent with all departments authorization note shall generated.
7.18.2 This shall be procedurelized by generating an authorization note by QA and getting acceptance / approval of all the concerned departments. The acceptance / approval can be sought through electronic mails. Printout of electronic mails shall be attached with authorization note.
7.18.3 Final approval of authorization note shall be given by Head-QA.
7.19 Record all the details of incidents in Incident Report Register as per Format
8.0 ABBREVIATIONS
SOP : Standard Operating Procedure
CAPA : Corrective & Preventive Action
QA : Quality Assurance
9.0 ANNEXURE
Annexure-I Incident Report
Annexure-II Incident Report Register
Annexure-I