sop for storage and destruction of Batch Manufacturing Records
1.0 OBJECTIVE
1.1 The objective of this SOP is to define the procedure for issuance, receipt, reviewed, storage and destruction of Batch Manufacturing/Packing Records.
2.0 SCOPE
2.1 This SOP is applicable for issuance, receipt, reviewed, storage and destruction of BMRs/BPRs of products being manufactured
3.0 RESPONSIBILITY
3.1 Asst. Officer or above –Quality assurance-Prepare the SOP and follow-up the SOP accordingly.
3.2 Asst. manager –Quality assurance -Provide the support to the implementation of SOP and maintained the records.
4.0 ACCOUNTABILITY
4.1 Head – Quality Assurance
5.0 REFERENCE(S)
5.1 In-House
6.0 PROCEDURE:
6.1 ISSUANCE
6.1.1 The Production personnel shall submit the form titled “Requisition for Issuance of BMR/BPR” as per Format well in advance (at least before one day) to QA as per production planning.
6.1.2 All the details related with product to be mentioned in requisition form.
6.1.3 As per the requirement, QA personnel shall arrange photocopy of the BMR/BPR from master BMR/BPR and affix controlled copy stamp in green colour on each page of BMR/BPR with sign & date.
6.1.4 QA person shall assign Batch Number as per the SOP titled ‘Batch Numbering System’ (SOP ) which shall be written by hand on each page at specified location, manufacturing and expiry date mentioned on the front page of BMR/BPR and also mentioned on store indent cum issue slip, Manufacturing and Expiry date for a certain batch having manufacturing date January 2015 and expiry date December 2017 shall be written as 01/2015 and 12/2017.
6.1.5 All the documents/ formats comprising the unfilled BMR shall be placed in a conventional file, before issuance to production.
6.1.6 All the details of the issuing BMR shall be entered in the records titled “BMR/BPR Issuance and Retrieval Record” as per Format
6.1.7 The unfilled BMR shall be sent to Head – Production or his / her designee and the receipt shall be obtained on record titled “BMR/BPR Issuance and Retrieval Record” as per Format
6.1.8 In case the batch is not in execution for more than 15 days, then the BMR of that batch shall be send back in QA by the Production personnel along with a inter office memo duly signed by Head-production or his/her designee.
6.1.9 In case of any change in production plan against any exigency like a change in market requirement or spoilage of page/s in the BMR/BPR, the same shall be returned to the QA along with a request for re-issuance of a fresh copy either of the spoilt page/s or the whole unfilled BMR on form titled “Extra Page Issuance Form” as per Format
6.1.10 QA personnel shall re-issue the required page/s of the BMR/BPR or in whole unfilled BMR against the request forwarded by Production. Necessary corrections /entries shall be made in the BMR/BPR Issuance Register.
6.1.11 The spoilt page/s or whole BMR/BPR shall be destroyed by QA.
6.1.12 The concerned production, stores, QC and IPQA personnel shall fill the unfilled BMR/BPR for specified process during the manufacture of drug product.
6.2 REVIEW& RECEIPT OF FILLED BMR/BPR:
6.2.1 After completion of manufacturing and packing process, filled BMR/BPR shall be reviewed by Head – Production or his/ her designee.
6.2.2 Head Production / deputy should ensure the completion of all batch documents before submitting to QA.
6.2.3 Completed batch document duly signed by head production along with IPQA data, finished product release report, finished goods transfer note and other related data if any to be sent to QA.
6.2.4 Head Production or his/her designee shall send the filled BMR/BPR to Head-QC for further review and signing.
6.2.5 Head – QC or his/ her designee shall review the BMR/BPR and sign with date and send to Head QA for approval and releasing of product for dispatch and sale.
6.2.6 Authorized person in QA will receive, review the BMR/BPR thoroughly & record entries.
6.2.7 Any discrepancy in process, equipment and yield or parameters which may have impact on yield / quality / safety of drug product should be critically examined. Photocopies of deviation / OOS reports should be attached along with BMR/BPR.
6.2.8 Emphasis to be given on following during BMR review.
6.2.9 General checks as per the check list but checks are not limited to following:
6.2.9.1 Records are submitted timely to QA after completion of batch by reviewing IPQA.
6.2.9.2 Error free coding details (Batch No., Mfg Date, Exp date, Mfg License no., MRP etc).
6.2.9.3 Tidy / Neat / Legible.
6.2.9.4 Corrections / Overwriting (if any duly signed and dated).
6.2.9.5 Timely entries.
6.2.9.6 Deviation reports if any.
6.2.9.7 Signature
6.2.9.8 Completion.
6.2.9.9 Verification.
6.2.9.10 Material reconciliation
6.2.9.11 For regular / part batch – Completion
6.2.9.12 IPQA data – Verification
6.2.9.13 Destruction record – Verification
6.2.9.14 Labels reconciliation – Verification
6.2.9.15 Overprinting matter – Verification
6.2.9.16 Yield at all stages – Verification
6.3 Head – QA shall ensure.
6.3.1 Completion of all documents.
6.3.2 Compliance of all corrections.
6.3.3 Compliance of all deviations.
6.3.4 Yield analysis.
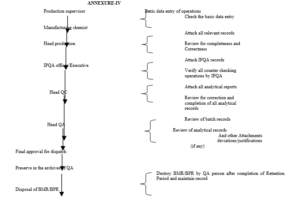
6.4 The flow diagram for handling of BMR/BPR shown as per Format
6.5 STORAGE
6.5.1 All the released BMRs/BPRs shall be filed and archived in Document Room (QA), Product wise and as per Batch No. with mfg. and exp. Details.
6.5.2 The BMRs/BPRs shall be preserved for five years or one year after the expiry date after that the BMRs/BPRs shall be destroyed by QA.
6.6 DESTRUCTION
6.6.1 QA personnel shall assess the due date for destruction of BMR/BPR(s) and prepare a list of BMRs/BPRs for destruction.
6.6.2 The BMR/BPR for destruction shall be destroyed by cutting in four pieces and burn with fire.
6.6.3 QA personnel shall maintain the destruction record of BMR/BPR.
7.0 ABBREVIATIONS
SOP : Standard Operating Procedure
QA : Quality Assurance
QC : Quality Control
BMR : Batch Manufacturing Record
BPR : Batch Packing Record
NA : Not Applicable
8.0 ANNEXURE
| Annexure No. | Title of Annexure |
| Annexure-I | Requisition for Issuance of BMR/BPR |
| Annexure-II | BMR/BPR Issuance and Retrieval Record |
| Annexure-III | Extra Page Issuance Form |
| Annexure-IV | Flow Diagram for Handling of Batch Manufacturing/Packing Record |
ANNEXURE-I
Date:
| To,
Quality Assurance Department |
From,
Production
|
Dear Sir,
Kindly issue Batch Manufacturing Record/Batch Packing Record for the following products:
| S. No. | Product Name | Batch
No. |
Batch
Size |
Mfg. | Exp. | Pack
Size |
BMR or BPR | Remarks |
ANNEXURE-II

ANNEXURE-III
| S. No. | Product Name | Batch
No. |
Batch
Size |
Mfg. | Exp. | Page
No. |
BMR or
BPR |
Remarks |
ANNEXURE-IV