Collection Storage Periodic Observation and Destruction of Raw Material and Finished Product Reference Samples
1.0 OBJECTIVE :
1.1 To lay down a procedure for collection and storage of raw material and finished product reference samples as well as periodic observation of Finished Product Reference sample.
2.0 SCOPE :
2.1 This procedure is applicable to all approved raw material used in manufacturing and marketed finished products being manufactured and packed at abc company.
3.0 RESPONSIBILITY :
3.1 Officer/Executive QC shall be responsible for collection, storage, periodic observation and destruction of raw material reference samples.
3.2 Officer/Executive QA shall be responsible for collection, storage, periodic observations and destruction of finished product reference samples of all batches manufactured at Fast Pharma Pvt. ltd. Roorkee.
4.0 ACCOUNTABILITY :
4.1 QC Manager / QA Head.
5.0 PROCEDURE :
5.1 COLLECTION OF REFERENCE SAMPLES
5.1.1 QC Officer/Executive shall collect the reference samples from each batch of raw materials in appropriate container after the sampling of raw material .
5.1.2 IPQA Officer/Executive shall collect the reference samples from each batch of finished products during packing process.
5.1.3 IPQA officer/Executive shall collect the samples randomly during in process checking of packing process so as the collect reference samples represent the whole batch. Total number of samples to be collect is mention in Annex-V.
5.1.4 The Finished Product Reference sample to be collected at least twice the quantity necessary for all tests required to determine whether the finished product meets its established specifications, including the quantity required for physical verification after every six month from manufacturing date till end of shelf life.
5.1.5 The raw material reference sample shall be collected in a quantity sufficient to carry out all the tests, except sterility and pyrogens / Bacterial endotoxins tests.
5.1.6 IPQA Officer/Executive shall collect separate samples for different packing style mentioned as below. In this case, total quantity to be collected should be equally divided among all the packing styles. If packed in more than one type of printed/non-printed packing materials. As example, packed as ‘Sale’ and ‘Physician Samples’ etc.
5.1.7 IPQA Officer/Executive shall check the over coding details i.e. B. No., Mfg. Date, Exp. Date, Price, etc. on packing materials against first page of Batch Packing Record.
5.1.8 IPQA Officer/Executive shall make entry into Refrence sample collection Register of finished product (Annex–I) and sign in it as well as into BPR of respective product.
5.1.9 Reference samples shall send to reference sample storage area.
5.1.10 Officer/Executive QA responsible for reference sample storage shall receive the reference sample and sign in register at received by column. He/she shall also verify the over coding detail and quantity of reference samples and if found satisfactory, shall make entry into Reference sample storage Register for finished product (Annex-II) and Raw Materials Reference Sample Inward, Storage and destruction register (Annex-IV).
5.2 STORAGE OF REFERENCE SAMPLES
5.2.1 Paste the sticker “REFERENCE SAMPLE/ CONTROL SAMPLE” on each pack and place in corrugated box and store under label claim storage condition as per below guideline:
5.2.2 Retain the Reference sample for 1 year after the expiration date of the drug product i.e. finished product. Reference sample of raw material destroy after 5 year from the month/year of GRN or after 2 year from its shelf life whichever is higher.
5.2.3 In case of requirement of reference samples by any head of the department, respective head of department shall fill ‘Requisition for issuance of Reference Sample’ (Annex-III) and get it approved by QA Head.
5.2.4 After QA Head approval, Officer/Executive QA(Reference sample) shall issue the required quantity of Reference Sample and record it in ‘Reference Sample storage register for Finished product.”(Annex–II).
5.3 PERIODIC OBSERVATION OF FINISHED PRODUCT REFERENCE SAMPLES
5.3.1 Carry out the periodic observation by visual inspection of reference samples at every 6 months from manufacturing date and up to end of shelf life of the finished product.
5.3.2 During periodic observation all the reference samples received from production shall be verified for physical parameters
| Sr. No. | Dosage Form | Parameters to be inspected |
| 1. | Small volume parenteral (liquid in ampoules) | Colour of solution and clarity ( w.r.t. particulate matters* / precipitates/glass particles)
|
| 2. | Liquid Syrup | Colour of solution and clarity ( w.r.t. particulate matters* / precipitates)
|
| 3. | External Preparation | Appearance, Leakage |
5.3.3 For External preparation take 01 unit and shall be checked.
5.3.4 Fill the physical observation into Annex-II by writing ‘OK’ if observation of required parameters is satisfactory.
5.3.5 Maintain a register for the consumption of Retain sample for physical observation having Sr. No., Name of Product, Batch Number, date of receipt, sample quantity receipt, physical observation frequency and remark columns.
5.3.6 In case of any abnormal observation, fill the abnormal reference samples observation report as Annex-VI and make star marking on same frequency in retain sample physical observation register.
5.3.7 Investigate the reason for abnormal observation.
5.3.8 Based on investigation QA head may decide following & write the same in QA remark column.
-Recall of batch/batches -Reformulation request to F&D
-Change in specification -Discontinuation of product
-Others
5.4 DESTRUCTION OF REFERENCE SAMPLE
5.4.1 Destruction of expired reference samples (i.e. 1 year after the expiration date of finished product) . the list approved by QA Head after verification of all the items for the destruction of the reference samples.
5.4.2 Withdraw & verify the samples as per the approved list.
5.4.3 Destroy the reference samples as per respective SOP.
5.4.4 Cleaning of control sample area also shall be recorded as per Annex -VII
6.0 ABBREVIATION :
| Sr. No. | Abbreviation used | Full form of abbreviation used |
| 1. | SOP | Standard Operating Procedure |
| 2. | QA | Quality Assurance department |
| 3. | B. No. | Batch Number |
| 4. | Mfg. Date | Manufacturing Date |
| 5. | Exp. Date | Expiry Date |
| 6. | HOD | Head of Department |
| 7. | w.r.t. | With respect to |
| 8. | GRN | Goods receipt Note |
7.0 ATTACHMENTS (ANNEXES) :
Annex-I : Reference Sample Collection register of Finished Product
Annex-II : Reference Sample Storage register for Finished Product
Annex-III : Requisition for issuance of Reference Samples
Annex-IV : Raw Material Reference sample inward, storage and destruction
Annex-V : Quantity to be collected as reference sample & Stability Sample
Annex-VI : Abnormal reference sample observation report
Annex-VII : Control Sample Cleaning Record
8.0 REFERENCE :
| Sr. No. | Reference Title |
| 1.0 | Schedule M (Good Manufacturing Practices and Requirements of Premises, Plant and Equipment for Pharmaceutical Products) |
| 2.0 | Quality assurance of Pharmaceuticals, A compendium of guidelines and related materials, Volume 2 |
| 3.0 | Guide to Good Manufacturing
Practice for Medicinal Products |
| 4.0 | 21 Code of Federal Regulations, Part 211 – Current Good Manufacturing Practice for Finished Pharmaceuticals |
Annex-I
Reference Sample Collection register of Finished Product

Annex-II
Reference Sample Storage register for Finished Product

Annex-III
Requisition for issuance of Reference Samples

Annex-IV
Raw Material Reference sample inward, storage and destruction

Annex-V
Quantity to be collected as reference sample and Stability Sample

Annex-VI
Abnormal reference sample observation report
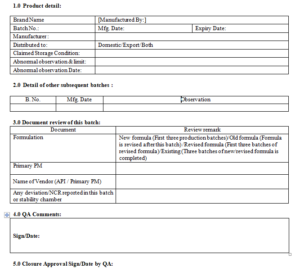
Annex-VII
Control Sample Cleaning Record

sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipment
concurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection