Validation for cleaning procedure liquid injection
| SCOPE OF VALIDATION | LIQUID INJECTION |
| PROTOCOL NO. | |
| SUPERSEDES | |
| EFFECTIVE DATE |
TABLE OF CONTENTS
| Sr. No. | SECTION TITLE | PAGE No. |
| 1.0 | PROTOCOL APPROVAL | |
| 2.0 | OVERVIEW | |
| 2.1 | OBJECTIVE | |
| 2.2 | PURPOSE | |
| 2.3 | SCOPE | |
| 2.4 | RESPONSIBILITY | |
| 2.5 | REVALIDATION | |
| 2.6 | CLEANING TYPES | |
| EQUIPMENT DETAILS | ||
| EXPERIMENTAL PLAN – CLEANING PROCEDURE | ||
| CHECK LIST FOR CLEANING PROCEDURE | ||
| 2.7 | APPROACH | |
| 2.8 | EXECUTION OF PLANNING | |
| 3.0 | CLEANING VALIDATION PROCEDURE | |
| 3.1 | LIST OF PRODUCTS | |
| 3.2 | WORST CASE SELECTION BASED ON SOLUBILITY STUDIES | |
| 3.3 | CLASSIFICATION OF PARTS | |
| 3.4 | SAMPLING TECHNIQUES | |
| 3.4.1 | SWAB SAMPLING -EXPERIMENTAL PLAN | |
| 3.4.2 | WASH WATER SAMPLING – EXPERIMENTATION PLAN | |
| 3.5 | VALIDATION ACCEPTANCE CRITERIA | |
| 4.0 | DETERMINATION OF ACCEPTANCE CRITERIA | |
| 4.1 | VISUAL CLEANLINESS CRITERIA | |
| 4.2 | DETERMINATION of acceptance criteria | |
| 4.2.1 | Calculation of MAC for Swab Sample | |
| 4.2.2 | CORRELATION OF SWAB SAMPLING WITH WASH WATER SAMPLING | |
| 4.3 | 10 PPM CRITERIA | |
| 4.4 | FINAL LIMIT | |
| 5.0 | CLEANING VALIDATION METHODOLOGY | |
| 5.1 | LABORATORY PROCEDURE RATIONALE | |
| 5.1.1 | BRIEF OUTLINE OF PROCEDURE | |
| 5.1.2 | SAMPLING PROCEDURE RATIONALE | |
| 5.1.3 | EXTRACTION PROCEDURE RATIONALE | |
| 5.1.4 | ANALYTICAL DETECTION RATIONALE | |
| 5.1.5 | RELATED DOCUMENTS | |
| 5.2 | EXPERIMENTAL PLAN | |
| 5.2.1 | EQUIPMENTS AND MATERIALS | |
| 5.2.2 | GENERAL INSTRUCTIONS | |
| 5.2.3 | PRE TREATMENT OF SWABS | |
| 5.3 | STABILITY OF API WORKING STANDARD SOLUTION | |
| 5.4 | BLANK SWAB INTERFERANCE ANALYSIS | |
| 5.5 | LINEARITY | |
| 5.5.1 | LINEARITY OF API WORKING STANDARD SOLUTION | |
| 5.5.2 | LINEARITY OF SPIKED SWAB EXTRACT | |
| 5.5.3 | RECOVERY OF SPIKED SWAB EXTRACT | |
| 5.6 | UV SPECTROPHOTOMETER METHOD | |
| 5.7 | RATIONALE FOR ESTABILISHING ACCEPTANCE PASS/FAIL RESIDUAL CONTAMINATION LIMITS OF API | |
| 5.8 | DOCUMENTATION | |
| 6.0 | DRAWING |
1.0 PROTOCOL APPROVAL
APPROVAL
Approval of this validation protocol will be joint responsibility of the following functional areas:
PREPARED BY:
| Department | Quality Assurance |
| Name | |
| Designation | Executive QA |
| Signature | |
| Date |
CHECKED BY:
| Department | Quality Control | Production |
| Name | ||
| Designation | Executive QC | Plant Head |
| Signature | ||
| Date |
APPROVED BY:
| Department | Quality Assurance |
| Name | |
| Designation | QA MANAGER |
| Signature | |
| Date |
2.0 OVERVIEW
2.1 OBJECTIVE
To validate the adequacy of the cleaning procedure currently being followed for equipment and to prove that the process is capable of removing any significant residues of previous batch.
2.2 PURPOSE
Cleaning procedure of equipment as per the Standard Operating Procedure needs to be validated to establish documentary evidence that the existing cleaning procedure is effective and avoids cross contamination.
2.3 SCOPE
This protocol is prepared to validate the cleaning procedure of equipment used for the manufacturing of sterile Liquid Injectables products.
2.4 RESPONSIBILITY
2.4.1 The validation team shall be responsible for overall compliance with this protocol.
(a) Monitoring of protocol completeness, accuracy, technical excellence and applicability
(b) Scheduling of validation
(c) Conducting of validation
(d) Data compilation and review
(e) Validation reports preparation and recommendation thereafter (if required)
2.4.2 The Production department shall be responsible for
(a) To provide all applicable cleaning and operational procedures, drawings and documentation necessary for the generation of this protocol
(b) To prepare system for validation
(c) To provide personnel to assist in the preparation and execution of this protocol
(d) To approve implement QA recommendations of deviations
2.4.3 The Quality Assurance (QA) department shall be responsible for
(a) To monitor the cleaning validation and carry out the sampling as per specified in the cleaning validation protocol for active residue analysis.
(b) To confirm and conduct cleaning validation by challenging the cleaning procedure with requirements as specified in approved validation protocol. Also to compile and review the data / report.
2.4.4 The Quality Control (QC) department shall be responsible for
(a) To provide all applicable methodologies, analytical procedures and documentation necessary for generation and execution of this protocol,
(b) To collect all analysis data,
(c) To complete and document apparatus calibration and methods validation,
(d) To provide personnel to assist in the preparation and execution of this protocol,
2.5 REVALIDATION
Cleaning procedure to be revalidated on:
Substitution of existing equipment with new equipment.
Any major modification in the existing equipment.
Any change in Standard Operating Procedure of Cleaning.
If Introduction of new product / manufacturing process which affects the acceptance criteria calculation.
Significant failures of Swab sample/ wash water analysis results.
Once in two years.
2.6 CLEANING TYPES
TYPE “A” CLEANING PROCEDURE
It involves cleaning of contact parts of equipment to a level of visual cleanliness. Type “A” Cleaning Procedure doesn’t need to be validated as there is no chance of cross contamination. It involves
• Changeover from one batch to next batch of the same product with same potency.
• Change over from one batch to next batch of the same product with higher potency.
Follow respective SOP for cleaning procedure.
TYPE “B “CLEANING PROCEDURE
It involves thorough cleaning of the contact and Non-Contact parts of equipment as per respective cleaning procedure. Finally the cleaned equipment shall be rinsed with defined volume of water for injection and then taking a swab/ wash water sample to assure a level of chemically clean.
• Changeover of one batch to next batch of the same product with descending potency.
• Change over of one batch to next batch of the different product with different actives or colour.
• After any maintenance of contact parts.
Follow respective SOP for cleaning procedure
EQUIPMENT DETAILS
a) Difficult to clean
b) Difficult to inspect
c) Contours of the vessel, which is likely to have residues build-up.
Experimental Plan – Cleaning Procedure
Dismantle the vessel by opening the lid from the top.
Remove the gasket, opening ports, and the S.S. pipe.
Allow the D.M.Water to flow for a few minutes before using it for cleaning.
Wash the vessel, gasket, opening ports, and the S.S. pipe with D.M Water by applying a jet with flexible hosepipe. Repeat this process twice for specific time.
Rinse the vessel, gasket, opening ports, and the S.S. pipe with (Respective quantity for specific equipment as per the SOP) WFI by applying a jet with flexible hosepipe.
Inform the IPQA Chemist to collect the water wash sample
Assemble the gasket, opening ports, S.S.pipe and lid after draining out all the water.
Apply the stretchable shrink-wrap film on the top openable parts.
Attach the equipment status label on the vessel.
Check and tick mark the checklist and do the required entries.
Repeat this cleaning procedure for three different batch runs.
Compile and evaluate three validation run results and revise the existing SOP, if required.
Check List For Cleaning Procedure
Previous Product Date:
Product : ____________________________________________
Batch No : ____________________________________________
| Sr. No. | CLEANING STEPS | STATUS |
| 1. | Dismantle the vessel by opening the lid from the top. | |
| 2. | Remove the gasket, opening ports, and the S.S. pipe. | |
| 3. | Allow the D.M.Water to flow for a few minutes before using it for cleaning. | |
| 4. | Wash the vessel, gasket, opening ports and the S.S. pipe with D.M. Water by applying a jet with flexible hosepipe. Repeat this process twice for specific time. | |
| 5. | Rinse the vessel, gasket, opening ports and the S.S. pipe with WFI (respective quantity for specific equipment as per the SOP) by applying a jet with flexible hosepipe. | |
| 6. | Inform the IPQA Chemist to collect the water wash sample | |
| 7. | Assemble the gasket, opening ports, S.S. pipe and lid after draining out all the water. | |
| 8. | Dry the vessel completely by circulating steam into jacket. | |
| 9.. | Apply the stretchable shrink-wrap film on the top of openable parts. | |
| 10. | Attach the equipment status label on the vessel. |
Remarks:
| Cleaned By Operator | Checked By Prodn. In charge | |
Name |
||
| Signature | ||
| Date |
Note:
Tick mark (√) – Completion of Job as per instruction
Cross mark (X) – Incomplete or incorrect Job done
2.7 APPROACH
• Preparation of SOP for the cleaning of the equipment.
• Establishing cleaning validation acceptance criteria by using following three
Concepts:
Visual Cleanliness
10 ppm Criteria
Dose Criteria
The strictest acceptance criteria value will be selected for cleaning validation. Acceptance criteria will be corrected based on the swab recovery; hence swab recovery test shall be carried out.
• Two type of sampling is planned for the detection of residual content of selected drug i.e., Swab Sampling and Wash Water Sampling.
• Only Chemical residual content will be validated in Cleaning Validation study. Microbiological residual content are not in the scope of this Validation protocol. Microbiological residual content cleaning validation is defined in Sterilized Material Hold Time Validation study.
• Testing method of Swab / Wash Water sample will be validated.
• Acceptance criteria shall be established for the swab sample analysis for the detection of residual content of selected drug as per Annexure III Matrix Calculation in terms of mcg/4 sq. inch of swab.
• Acceptance criteria shall be established for the wash water sample analysis for the detection of residual content of selected drug as per Annexure III Matrix Calculation in terms of mcg/ml. Wash water analysis limit for selected drugs shall be calculated for each equipment/vessel/accessories and the minimum limit shall be taken as final acceptance criteria for wash water sample analysis.
• Routine product change over cleaning will be evaluated based on the Wash Water sample result.
• Three qualifications run will be carried out with three cleaning run for any worst case (least soluble drug).
• Cleaning validation shall be carried out with worst-case product selected from the product grouping manufactured. Product Matrixing is defined in Annexure I.
• Worst-case selection shall be done based on the least solubility of the product residue in water. If all products are equally soluble in water any one of the product will be selected for validating the SOP of the equipment cleaning.
• If all data comply the acceptance criteria, SOP shall be considered as validated for all the product change over and batch-to-batch cleaning.
2.8 EXECUTION OF PLANNING
• Pre validation study:
List of Products being manufactured.
Worst case selection for cleaning validation study on the basis of
Solubility
Worst case selection on the basis of Equipment, classification of the parts of equipment.
Selection of sampling technique
Determination of acceptance criteria
• Validation runs:
Three successful runs of a standard process to establish a validated cleaning
Programme.
• Sampling and testing
• Collection of data
• Reporting of the results
• Evaluation of the results
• Preparation of validation report
3.0 CLEANING VALIDATION PROCEDURE
3.1 (A) LIST OF PRODUCTS (IN AMPOULES):
| S. No. | Product Name | Strength (mg/ml) |
| 1. |
|
Vitamin B1- 100 mg/ml
Vitamin B6- 100 mg/ml Vitamin B12- 1.0 mg/ml Nicotinamide- 100 mg/ml D-Panthenol- 50 mg/ml |
(B) LIST OF PRODUCTS (IN VIALS):
| S. No. | Product Name | Strength (mg/ml) |
3.2 WORST CASE SELECTION BASED ON SOLUBILITY STUDIES
Water is used as solvent for the cleaning of equipment. A solubility study in water is to be carried out for the validation.
| Sr. No. | Product Name | Solubility in water | ||||
| Very soluble | Freely soluble | Soluble | Sparingly Soluble
|
Insoluble | ||
* Actout (Ivermectin) is selected as worst case product on the basis of Solubility and Dose strength criteria in non-aqueous category.
** Fabocal (Cholecalciferol) is selected as worst case product on the basis of Solubility and Dose strength criteria in aqueous category.
As per above table, a number of products are sparingly soluble and practically insoluble in water. Therefore out of these selections of two molecules, that is one from aqueous (Fabocal) and one from non-aqueous (Actout) category during cleaning validation provides sufficient data for validation of cleaning procedure.
3.3 CLASSIFICATION OF PARTS
Contact Parts:
The contact parts are those, which will come in contact with the product. Attention to be taken while cleaning and swabbing the Hard to clean parts. The Hard to clean parts those, which are difficult to clean and difficult to inspect like contours of the surfaces, which are likely to have residues buildup.
Non-Contact Parts:
The non-contact part shall be listed under this head, which shall be cleaned as per the respective SOP’s
3.4 SAMPLING TECHNIQUES
Following sampling technique shall be followed for cleaning validation studies
Swab sampling
Wash Water sampling
3.4.1 SWAB SAMPLING – EXPERIMENTAL PLAN
Swab sampling technique for drug contamination:
• Wear the hand gloves
• Pipette out 10ml of sampling solvent in transport container bearing identification label.
• Remove a swab from its protective bag using a clean latex hand glove.
• Avoid unnecessary touch to swab tip to prevent from contamination.
• Transfer the swab in transport container (test tube) containing 10ml of sampling solvent and allow the swab to soak completely.
• Take out the swab from sampling solvent and squeeze the tip (by means of SS forceps) against inner surface of test tube to remove excess solvent in such a manner that excess sampling solvent drips inside the test tube.
• Hold the stem of swab without touching the head of swab.
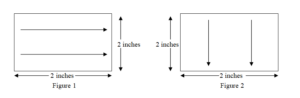
• Using one side of moistened swab wipe the test surface of 2” x 2” with 10 firm horizontal strokes as illustrated in figure 1. Wherever 2” x 2” area is not available for swab sampling, carry out sampling from hardest to clean area.
• At the end of the each stroke lift the swab carefully.
• Turn the swab over to its other side, wipe the test surface of 4” x 4” with 10 firm vertical strokes as illustrated in figure 2
• At the end of the each stroke lift the swab carefully.
• Cut the handle of swab and put in transport container having solvent.
 .
.
Swab sampling technique for microbial evaluation: Refer relevant microbiology department SOP for swab sampling for microbial evaluation.
3.4.2 WASH WATER SAMPLING – EXPERIMENTATION PLAN
Wash water sample shall be collected after rinsing the equipment with specified quantity of water for Injection as defined in individual protocol.
The samples taken shall be analyzed for the content of residual Active ingredient to establish that the residual levels after cleaning are below the set acceptance criteria.
3.5 VALIDATION ACCEPTANCE CRITERIA
Type ‘A’: No visual accumulation of the previous batch
Type ‘B’: The residual active ingredient in the final Swab sample/Wash Water sample collected after cleaning of the equipments should not be greater than acceptance criteria.
4.0 DETERMINATION OF ACCEPTANCE CRITERIA
4.1 VISUAL CLEANLINESS CRITERIA
Visual cleanliness criteria shall be the primary criteria for the cleaning of equipment. Based on the data, visually clean surface contains the product residual less than 100 mcg/4 square inches (Pharmaceutical Technology, October 1998, “Establishing scientifically Justified Acceptance Criteria for cleaning Validation of Finished Drug Products”.
4.2 DETERMINATION OF ACCEPTANCE CRITERIA FOR RESIDUAL CONTAMINATION
4.2.1 Calculation of MAC for Swab Sample (Dose criteria)
• The acceptance criteria for residual drug contamination is determined as MAC (Maximum Allowable carry over)
• Maximum Allowable carry over (MAC) of previous product in the subsequent product is calculated by using the formula mentioned below,

• Maximum allowable carry over is calculated considering worst case product as previous product and entire range of products as next product (minimum batch size) using above formula. Individual MAC limits obtained for all products are tabulated with all permutations of previous product in Annex-III. The lowest value out of this is selected as minimum MAC.
• The least value out of all minimum MAC shall be considered as acceptance limit for all products.
• Assuming the contamination is present uniformly on the entire contact surface area, the limit for one square inch of surface is calculated by dividing above MAC value by total contact surface area in square inch.
Limit for one sq. inch= MAC/Total surface area in sq. inch
• Now the limit of residual contamination for a swab area of 2” x 2” can be calculated as follow,
The limit of residual contamination = Limit for one sq. inch x 4
• Equipment covered under cleaning validation and calculation for acceptance criteria for finished products are illustrated and derived in Annex-II and Annex-III respectively.
4.2.2 CORRELATION OF SWAB SAMPLING WITH WASH WATER SAMPLING
Once the residue limit in the subsequent product is determined in terms of active ingredient contamination level per swab, the next step is to determine the residue limit per ml in case of Wash Water Sample. This limit can be calculated as
Residue limit per ml = MAC x Surface Area of equipment (in in2)
(μg/ml) Rinse Volume taken for equipment (in ml) x 4
4.3 10 PPM CRITERIA
Industrial Trend of Using default limit of maximum allowable residue is 10 ppm, which is 10 mg per Kg of the next batch output.
RX(S/T)XV
The Maximum allowable residue per swab of 2 X 2 in2 can be mathematically expressed as
R = 10mg active ingredient in product A /kg of product B
S = Number of kilograms per batch of final mixture of product B
T = equipment surface area in common between products A and B expressed as square inches.
U = 4 in.2 swab
Factor R is always 10mg of active ingredient of the product being cleaned per kilogram of final mixture of the recipient product. This is simply 10ppm stated a different way.
Factor S appears in the numerator. Residues of product A appearing in product B will be diluted by product B. a larger batch size of product B will diluted the residue more and therefore a larger limit is acceptable for product A.
Factor T is the number of square inches of product-contact surface area in common between the two products as before.
Factor U is the standard 4 in.2 swab as before.
4.4 FINAL LIMIT
This minimum value from all three criteria shall be considered as acceptance limit for product A into product B.
After considering all the three criteria (Visually Clean Criteria, Dose Criteria and 10ppm Criteria), the acceptance limit for cleaning validation shall be finalized.
The finalized limit shall be valid as long as equipment used & product combination manufactured in the facility remains same.
If any new product is added, the cleaning limits to be recalculated.
5.0 CLEANING VALIDATION METHODOLOGY
• Ensure that operator for cleaning are trained
• Clean the equipment as per respective SOP after the processing of the product.
• Collect the swab samples from the location described in Annex-II.
• Simultaneously collect the Rinse sample for analysis purpose.
• Send the sample to QC for analysis with Analysis request form.
• QC analyst shall perform the test as per the STP and record the result in the data sheet.
• Prepare the report of the sample test result.
• Evaluate the results of the compliance with the acceptance criteria.
• Prepare the Validation report.
5.1 LABORATORY PROCEDURE RATIONALE
5.1.1 BRIEF OUTLINE OF PROCEDURE
Laboratory procedure is a limit test performed on HPLC. The swab sampling of cleaned equipment is done as per SOP. This procedure is used to validate the cleaning of equipments subsequent to use in manufacturing of products containing least soluble drug (for aqueous and non-aqueous). The method is to be validated for the drug at acceptance limit per 2 x 2 sq. inch test surface. Results are reported as pass or fail at the stated acceptance limit.
5.1.2 SAMPLING PROCEDURE RATIONALE
The appropriate diluting solvent will be used as sampling solvent with respect to their individual solubility profile of API (Active Pharmaceutical Ingredient).
Clean room laundered polyurethane foam tip swabs shall be selected as sampling medium due to their non-leaching and non-fibers shedding property. The swabs were pre-treated to get rid of any possible interference as precautionary measure.
5.1.3 EXTRACTION PROCEDURE RATIONALE
Based on the individual solubility of API, select the solvent for the extraction of drug from swabs.
5.1.4 ANALYTICAL DETECTION RATIONALE
Select the respective absorbance maxima of API.
5.1.5 RELATED DOCUMENTS
Swab sampling for validation of test surfaces analysis of API in swabs.
5.2 EXPERIMENTAL PLAN
5.2.1 EQUIPMENTS AND MATERIALS
a. High Performance Liquid Chromatography.
b. Clean room laundered polyurethane foam tip swabs with polypropylene Stick
c. S.S Plates
d. Transportation container (stoppered glass test tube)
e. Other glassware
f. API working standard
5.2.2 GENERAL INSTRUCTIONS
Utmost care to be taken during handling of swabs while sampling and analysis to avoid touching the swab with anything to prevent swab from contamination.
All glassware must be clean and dry.
5.2.3 PRE-TREATMENT OF SWABS
All the swabs to be used for method validation shall be previously treated as follows.
Take the required number of swabs in suitable capacity of stoppered conical flask and shake vigorously with approximately 150 ml Acetonitrile, draining the Acetonitrile then boil the swabs in 3x150ml of water (Volume of water can be changed as per requirement) for 5 minutes each time draining the water and ensure that there are no interfering substances present. Transfer the washed swabs to a clean glass tray and allow drying at 105˚ C for 60 minutes.
5.3 STABILITY OF API WORKING STANDARD SOLUTION
Under respective chromatographic conditions of API at determined detector response at assay concentration of API in injection, at time intervals of 0, 2, 4, 8, 24 & 48 hours at room temperature. Tabulate the detector response in Annex-IV.
Determine the maximum time period at which the detector response of solution remain stable with respect to zero hrs. (That is the time in which the detector response does not vary by more than 5% to that of 0 hrs.) This time period will become the time limit within which the standard solution will be used.
5.4 BLANK SWAB INTERFERENCE ANALYSIS
Take 3 previously treated swabs and dip the tips in 3 different test tubes containing known volume of extracting solvent. Allow standing for 15 minutes.
Shake the test tube vigorously. Extract the extracting solvent from the swabs and filter through 0.45μ filter paper and inject 20 µl and tabulate the detector response in Annex-V.
There should not be interference of substances with a peak of API.
5.5 LINEARITY
5.5.1 LINEARITY OF API WORKING STANDARD SOLUTION
Analyzing working standard solutions of suitable concentrations to establish that test results are directly proportional to the concentration of analyte in sample at given range assesses linearity of the laboratory procedure. Tabulate the results in Annex-VI and plot the graph of detector response V/s concentration. The resulting graph shall be straight line.
5.5.2. LINEARITY OF SPIKED SWAB EXTRACT
Prepare 3 standard solutions of different concentrations (should fall in linear range) from API working standard.
Take 3 swabs and apply to each of them known volume of respective standard solutions with help of graduated pipette. Touch the pipette on the tip of the swab and slowly deliver the solution by slightly pressing so as to absorb the solution properly by the swab.
These spiked swabs will contain known concentrations of the drug respectively.
Place these spiked swabs individually in stoppered test tubes containing know volume of extracting solvent. Stopper the test tube and shake vigorously to extract the drug in solution.
Filter the extracting solvent through 0.45μ filter paper and inject known volume and note the detector response.
Simultaneously measure the detector responses of corresponding standard solutions (Concentrations corresponding to that in swabs)
Calculate the API content in each swab and percentage recovery from each swab. Calculate mean percentage recovery and RSD. Tabulate the results Annex-VII.
To consider this experiment to be satisfactory the minimum recovery should be 80% and RSD should not be more than 10.0%.
Calculate mean detector response of each concentration and plot a graph of detector response Vs Concentration. It should be straight line.
5.5.3 RECOVERY OF SPIKED SWAB EXTRACT
Prepare 3 standard solutions of different concentrations (should fall in linear range) from API working standard.
Spike uniformly 2” x 2” surface of S.S.Plate with known concentrations of solution with the help of graduated pipette and allow the surface to dry at room temperature.
Swab each of the 2” x 2” surfaces treated as above using different swabs.
Place these swabs individually in stoppered test tubes containing known volume of extracting solvent. Stopper the test tube and shake vigorously to extract the drug in solution.
Filter the extracting solvent through 0.45 filter paper and inject known volume and note the detector response.
Simultaneously measure the detector responses of corresponding standard solution (Concentrations corresponding to that in swabs)
Calculate the API content and % recovery in each of the swabs. Calculate mean, % recovery and RSD and tabulate the results in Annex-VIII.
The recovery shall not be less than 80% and RSD shall not be more than 10%.
5.6 UV SPECTROPHOTOMETER METHOD:
Determine the linearity of working standard on UV spectrophotometer.
Compare between detector response and absorbance of API working standard.
5.7 RATIONALE FOR ESTABLISHING ACCEPTANCE PASS / FAIL
RESIDUAL CONTAMINATION LIMITS OF API:
Three criteria mentioned below were considered for establishing acceptance limit for residual contamination of API in test surface of 2″ x 2″.
a) Max. 1/10000th effective dose in maximum daily dose of subsequent product.
b) Max. 10 ppm in the subsequent batch.
c) Max. 100 mcg per swab of 2″ x 2″ test surface [which indicate visually clean].
5.8 DOCUMENTATION
| Annex No. | Formates |
| Annex I | Products covered under cleaning validation |
| Annex II | Equipment chain |
| Annex III | Determination of acceptance criteria |
| Annex IV | Stability of API working standard. |
| Annex V | Blank swab detector response for analysis of API |
| Annex VI | Linearity of API working standard solution. |
| Annex VII | Linearity and recovery in swab spiked with API |
| AnnexVIII | Recovery of API from swabs of spiked S.S.plates. |
| Annex IX | Swab analysis results |
| Annex X | Wash water analysis result |
| Annex XI | Swab Analysis Results By UV Method |
| Annex XII | Wash Water Analysis Result By UV Method |
6.0 DRAWING

process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipment
concurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection