process validation protocol for methylcobalamin niacinamide and pyridoxine injection
| SUPERSEDES PROTOCOL No. | |||
| DATE OF VALIDATION | |||
| VALIDATION BATCH NUMBERS | |||
| VALIDATION BATCH SIZE | |||
PROTOCOL CONTENTS
| S. NO. | TITLE |
| 1.0 | PROTOCOL APPROVAL |
| 2.0 | OBJECTIVE |
| 3.0 | SCOPE |
| 4.0 | RESPONSIBILITY |
| 5.0 | PRODUCT INFORMATION |
| 6.0 | PROCESS VALIDATION METHODOLOGY |
| 7.0 | REASON FOR VALIDATION |
| 8.0 | SITE OF STUDY |
| 9.0 | PROCESS VALIDATION PRE-REQUISITE |
| 10.0 | MANUFACTURING FORMULA |
| 11.0 | PROCESS FLOW DIAGRAM |
| 12.0 | PROCEDURE |
| 13.0 | REVALIDATION |
| 14.0 | VALIDATION STABILITY PLAN |
| 15.0 | REFERENCES |
| 16.0 | DOCUMENTS TO BE ATTACHED |
| 17.0 | NON COMPLIANCE, IF ANY |
| 18.0 | DEVIATION FROM PRE-DEFINED SPECIFICATION, IF ANY |
| 19.0 | CHANGE CONTROL, IF ANY |
| 20.0 | ABBREVIATIONS |
| 21.0 | REVISION HISTORY |
1.0 PROTOCOL APPROVAL:
PREPARED BY:
| DEPARTMENT | NAME | DESIGNATION | SIGNATURE/DATE |
| QUALITY ASSURANCE |
REVIEWED BY:
| DEPARTMENT | NAME | DESIGNATION | SIGNATURE/DATE |
| QUALITY ASSURANCE | |||
| ENGINEERING | |||
| PRODUCTION | |||
| QUALITY CONTROL |
APPROVED BY:
| DEPARTMENT | NAME | DESIGNATION | SIGNATURE/DATE |
| HEAD QUALITY ASSURANCE |
2.0 OBJECTIVE:
• To validate the manufacturing and packing process of Methylcobalamin, Niacinamide & Pyridoxine injection as per
the Master Formula Record to establish documentary evidence that the process will consistently produce the product,
meeting its predetermined In process and Finished Product Specification by examining (total no. of 03 batches) consecutive production batches.
3.0 SCOPE:
• The Protocol covers all aspects of Process Validation of Methylcobalamin, Niacinamide & Pyridoxine Injection manufactured
as per the Master Formula Record in Ampoule Line (Injection Block) at A B C Pharma
• Three consecutive production batches of Methylcobalamin, Niacinamide and Pyridoxine Injection manufactured as per its
Master Formula Record are to be validated as per this protocol.
• Report generated after performing sampling and testing is to be utilized for finalization of Master Formula Record as well as Batch Manufacturing and Packing Record.
4.0 RESPONSIBILITY:
• The Validation Group, comprising of a representative from each of the following departments, shall be responsible for the overall compliance of this Protocol.
| DEPARTMENTS | RESPONSIBILITIES |
| Quality Assurance | To Prepare, Review and Approval of Protocol
Protocol Training To monitor all validation activities and ensuring the validation as per the protocol. To monitor protocol completeness and technical accuracy To review and compile the data and approve the validated procedure, if found as per acceptance criteria. |
| Production | · To review of protocol.
· To schedule the validation activity. · To provide personnel to assist for execution of this protocol. |
| Quality Control | · To review of protocol
· To provide all applicable analytical procedures and documentation necessary for execution of this protocol. · To test the sample collected and provide all Analytical Data. |
| Engineering | · To review of protocol.
· Co-ordination, Execution and technical support in process validation activity. |
5.0 PRODUCT INFORMATION:
| Product Code | |
| Generic Name | Methylcobalamin, Niacinamide and Pyridoxine IP |
| Label Claim | Each 02 ml contains |
| Methylcobalamin IP………….1000 mcg | |
| Niacinamide IP……………….100 mg | |
| Pyridoxine IP…………………100 mg | |
| Water for Injection IP………….q. s. | |
| Standard Batch Size: | |
| Batch Size: | |
| Pack Size: | 02 ml |
| Primary Pack Description | 02 ml Amber Glass Ampoules with green ring |
| Pack sizes | 01 Ampoule per inner carton (Blister pack) |
| Shelf-life | 18 Months |
| Storage Condition | Store Protected from Light and Moisture.
Kept in cool and dry place |
| Product Description | Red colour Clear solution |
6.0 PROCESS VALIDATION METHODOLOGY:
• Only after validation protocol approval, the protocol shall be executed.
• During the course of validation the documentation systems, laboratory controls, in process checks during production shall be evaluated.
• The process validation methodology consists of three basic parts critical process parameters monitoring, the routine in process and final
product release testing & additional validation sampling and testing.
• The type of validation to be carried out is Concurrent Validation.
• The validation exercise for three batches of production scale shall be done to assess the process consistency.
• QA departments shall take samples as per approved Process Validation Protocol.
• All parameters shall be recorded in relevant records (e.g. Process Validation Report, Formats and BMR & BPR etc.)
• QC shall analyze all validation samples and the raw data shall be recorded / attached with the report. Where applicable the graph
and data print outs of critical process parameters shall be obtained and attached.
• Process Validation data shall be recorded in the process validation report and the recorded data will be identified by date.
• All documents related to process validation including batch specific information, certificates for test instrument used for analysis and
certificates for critical instruments of the processing equipment shall be attached to Process Validation Report.
• A final report shall be prepared summarizing the data obtained from the validation batches, conclusions drawn and recommendations, if any.
7.0 REASON FOR VALIDATION:
• New Product manufactured at Ampoule Line in ABC pharma
8.0 SITE OF STUDY:
• Injection Block at ABC Pharma
9.0 PROCESS VALIDATION PRE-REQUISITE:
• The batches shall be manufactured as per the batch manufacturing record / process.
• Three batches shall be taken for process validation study.
• Three consecutive successful runs shall be considered for completion of validation activity.
• The equipments used for manufacturing and processing of these batches shall be as per list of equipments.
Critical equipment/instrument must be in validated/qualified status being used during process validation.
• The raw material used for manufacturing shall be from approved vendors and shall be released by quality control.
• Sampling for in-process samples shall be carried as per established sampling procedure and plan.
• Critical in-process control shall be evaluated with respect to the laid down specification.
• Finished drug product of these batches shall be analyzed as per laid down test procedures and predetermined specifications.
9.1 Verification of Documents:
• Master document shall be approved, signed and dated by appropriate persons prior to commencement of batch processing.
• Verify the following master documents and record the details in Process Validation Report
a. Master Formula Record
b. Batch Manufacturing Record
c. Batch packing record
d. Process Validation Protocol and Report
e. Raw Material Specifications and Standard Testing Procedures
f. Packaging Material Specifications and Standard Testing Procedures
g. Finished Product Specification and Standard Testing Procedures
9.2 Training Record of Validation Team:
• All the personnel involved in the manufacturing and Packing of Validation Batches, Sampling and Testing of Validation Samples
should be appropriately trained both in their job related activities and on the process validation protocol by Head-QA.
• Verify the Training Records of the persons involved in the process validation activity and record the details in Process Validation Report.
9.3 Equipment Qualification Verification:
Ensure all equipments to be used for the manufacturing must be qualified. The reference Qualification Documents shall be verified and
mentioned in the Process Validation Report. The list of major equipments used for manufacturing of Methylcobalamin,
Niacinamide and Pyridoxine Injection IP in Ampoule line mentioned below:
| S. No. | Name of Equipment / Machine | Identification No. |
| 1. | Manufacturing Vessel | |
| 2. | Manufacturing Vessel | |
| 3. | Collection vessel | |
| 4. | Collection vessel | |
| 5. | Ampoule Washing Machine | |
| 6. | Depyrogenating Tunnel | |
| 7. | Ampoule filling & sealing Machine | |
| 8. | Laminar Air Flow Unit (filling & Sealing) | |
| 9. | Laminar Air Flow Unit (Cooling zone) | |
| 10. | Laminar Air Flow Unit (Filtration Room) | |
| 11. | Laminar Air Flow Unit (Filtration Room) | |
| 12. | Autoclave | |
| 13. | Pure Steam Generation System | |
| 14. | Water System (WFI) | |
| 15. | Dynamic Garment Storage Cabinet | |
| 16. | Dynamic Pass Box | |
| 17. | Dynamic Pass Box | |
| 18. | Dynamic Pass Box | |
| 19. | Mobile Laminar Air Flow | |
| 20. | Mobile Laminar Air Flow |
10.0 MANUFACTURING FORMULA:
10.1 Bill of Raw Materials:
| Sr. No. | Ingredient | Pharmacopoeial
Status |
Label
Claim |
Theoretical Quantity
(For 220 Liters) |
Units | Overages | Source of API vendor |
| 01. | Methylcobalamin | IP | 500 mcg | Kg. | 100 % | ||
| 02. | Niacinamide | IP | 50 mg | Kg. | 10 % | ||
| 03. | Pyridoxine Hcl | IP | 50 mg | Kg. | 10 % | ||
| 04. | Benzyl Alcohol | IP | – | Ltr. | – | ||
| 05. | Monothioglycerol | IP | – | Ltr. | — | ||
| 06. | Di Sodium EDTA | IP | – | Kg. | — | ||
| 07. | Sodium Hydroxide | IP | – | Kg. | — | ||
| 08. | Water for injection | IP | – | Q.S. | Liter | — |

10.2 Bill of Primary Packing Materials:
| Sr. No. | Name of Material | Theoretical Quantity
(For 220 Liters) |
Function | Unit | Source of PPM Vendor |
| 1. | 02 ml Amber Glass Ampoules with green ring | 100000 * | Primary
Packing Material |
Nos. |
* 5% excess quantity of material to compensate processing loss.
11.0 PROCESS FLOW DIAGRAM: (Manufacturing Process)
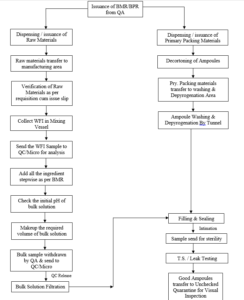
11.1 PROCESS FLOW DIAGRAM (Packing Process)
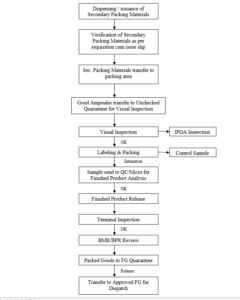
12.0 PROCEDURE:
12.1 SAMPLING METHODOLOGY:
| S. No. | STAGE | DEPARTMENT | PROCEDURE |
| 1. | Machine part Rinse/Swab | Production | Wash the Equipment’s as per the respective Cleaning Procedure. |
| QA | Monitor the process as per the BMR and withdraw the samples as per Sampling Plan. | ||
| QC | Timely complete the analysis and provide the results to QA | ||
| 2. | Cleaning and Sterilization of Equipment’s | Production | Wash the Equipment’s as per the respective Cleaning Procedure and Sterilize the washed equipments at 121.4ºC for 30 minutes. |
| QA | Monitor the process as per the BMR and cross verify Sterilization Charts. | ||
| QC | Timely complete the analysis and provide the results to QA | ||
| 3. | Water For Injection | Production | Take the WFI sample before bulk manufacturing from mixing tank. WFI Specification as pH – (5-7), Conductivity- NMT 1.3 µs/cm, BET- 0.25 EU/ml, Bio Burdon 10 CFU/100 ml, TOC NMT 500 PPB. |
| QA | Monitor the process as per the BMR and cross verify Sterilization Charts. | ||
| QC | Timely complete the analysis and provide the results to QA | ||
| 4. | Preparation of Bulk Solution | Production | Prepare bulk Solution as per step mentioned in Batch Manufacturing record. |
| QA | Monitor the process as per the BMR and withdraw the samples as per Sampling Plan. | ||
| QC | Timely complete the analysis and provide the results to QA | ||
| 5. | Filtration of Bulk Solution | Production | Perform the bubble point test of the membrane filter (pre integrity)
Start the filtration from Mixing vessel to collection vessel by applying 0.2 micron filtered Nitrogen gas pressure. Perform the bubble point test of the membrane filter (post integrity). |
| QA | Monitor the process as per the BMR & BPR and withdraw the samples as per Sampling Plan | ||
| QC | Timely complete the analysis and provide the results to QA | ||
| 6. | Ampoules washing | Production | Wash Ampoules sample collect during initial middle & end stage from washing area. |
| QA | Monitor the process as per the BMR & BPR and withdraw the samples as per Sampling Plan | ||
| QC | Timely complete the analysis and provide the results to QA | ||
| 7. | Sterilization and
Depyrogenated Ampoules |
Production | Set the temperature of heater as per validated set parameters and sterile the ampoule through ampoule sterilization Tunnel. In order to sterilize the ampoules at NLT 310°C. |
| QA | Monitor the process as per the BMR & BPR and withdraw the samples as per Sampling Plan | ||
| QC | Timely complete the analysis and provide the results to QA | ||
| 8. | Filling & Sealing | Production | Perform Filling and Sealing Operation as described in Batch Manufacturing Record. The syringe is calibrated for fill volume range of 1.05ml to 1.15ml after the uniformity is established, start the Filling and Sealing Operation. |
| QA | Monitor the process as per the BMR & BPR and withdraw the samples as per Sampling Plan | ||
| QC | Timely complete the analysis and provide the results to QA. | ||
| 9. | Visual Inspection of the Filled Liquid Ampoules | Production | Perform Visual Inspection of the filled Ampoules for any defects e.g. Scratches, Black or White Particles, sealing defect, Fiber & glass defect Volume Variation, etc. |
| QA | Monitor the process as per the BMR/BPR and withdraw the samples as per Sampling Plan | ||
| QC | Timely complete the analysis and provide the results to QA. | ||
| 10. | Packing | Production | After getting approval from IPQA Department, pack the Inspected Liquid Ampoules as per the packing style mentioned in the BMR/BPR. |
| QA | Monitor the process parameters as per the BPR and withdraw the samples as per the sampling plan.
QA shall also withdraw the samples for stability as per the sampling plan. |
||
| QC | Timely complete the analysis and provide the results to QA. |
12.2 PROCESS STEPS AND CRITICAL PROCESS VARIABLES FOR VALIDATION:
| PROCESS STEP | CRITICAL PROCESS VARIABLES |
JUSTIFICATION |
| Cleaning and Sterilization of Equipment’s | Cleaning of Machine parts/Equipments
Sterilization Time (30min.) Sterilization temperature (NLT 121.40C) |
The Process of check & ensure for Mixing Vessel, Collection Vessel and Machine parts Clean and sterilization. |
| Preparation of Bulk Solution | Mixing Time
Process Time Mixing Speed
|
The process of mixing shall be evaluated for homogeneity of the solution through the sampling of mixed materials from 2 locations (Top Layer & Bottom Layer) at different time interval i.e. After 05 minutes, after 10 minutes and after 15 minutes of mixing. Sample shall be collected from different locations and 1 composite sample. The Samples shall be submitted to QC As per bulk specification. |
| Filtration of Bulk Solution | Pre & Post Integrity of Filters
Process Time. |
The process of Filtration shall be evaluated through the sampling of filtered solution for determination of Sterility. Samples shall be collected by the trained Microbiologist and perform Sterility Test. |
| Ampoules washing | Recirculated water Pressure 1.5 to 2.0 Kg/cm2
Compressed Air Pressure 1.5 to 3.0 Kg/cm2 Purified Water Pressure 1.5 to 2.0Kg/cm2 WFI Pressure 1.5 to 2.0Kg/cm2 |
The process of Ampoules washing shall be evaluated through the sampling of ampoule washing at different stage mention below.
1. Initial 2. Middle 3. End |
| Ampoules Sterilization | Differential Pressure in (Pa)
· DP of Drying zone 05-15 mm of water · DP of Sterilization zone 10-25 mm of water · DP of Cooling zone 05-15 mm of water Temperatures in °C a. Drying zone b. Sterilization zone c. Cooling zone Speed of Conveyor (mm/ min) |
The process of Ampoules Sterilization shall be evaluated through the sampling of Ampoule Sterilization at different stage mention below.
1. Initial 2. Middle 3. End |
| Filling and Sealing | Presence of Particles
Process Time Machine Speed a. Minimum Speed (144 Ampoules/min.) b. Optimum Speed (192 Ampoules/min.) c. Maximum Speed (240 Ampoules/min.) |
The process of Filling and Sealing shall be evaluated through the sampling of Filled and Sealed Ampoules which is produced at different time points as mentioned below:
1. At the start of filling and sealing operation. 2. At the middle of Filling and Sealing Operation. 3. Towards the end of Filling and Sealing Operation. Samples shall be submitted to QC for testing as per finished product specification Sterility & BET. |
| Packing | Visual Inspection
Overprinting details Packing Style
|
Observation of Visual Inspection shall be check as per BPR.
During packaging process At Start, middle and towards end of packing parameter shall be check. Overprinting details Packing Style Check As per batch packing record |
12.3 SAMPLINGs AND ANALYSIS PLAN WITH ACCEPTANCE CRITERIA:
Collect the samples at various intervals at different operations as per the Sampling Plan mentioned below
| Sample | Location of Collection | Test to be performed | Acceptance Criteria | Responsibility |
| Machine parts Rinse/Swab (Whichever applicable) | From Equipment washing area | Description | Clear colourless free from particulate matter | QC/Micro |
| Clarity | Should be clear | |||
| pH | 5.0 to 7.0 | |||
| Conductivity | NMT 1.3 µs/cm | |||
| Previous product Residue | No residue of previous product | |||
| Bio Burdon | NMT 10 cfu/100 ml | |||
| Wash water of Cleaning | From Manufacturing tank, Collection vessel | Description | Clear colourless free from particulate matter | QC |
| Clarity | Should be clear | |||
| PH | 5.0 to 7.0 | |||
| Conductivity | NMT 1.3 µs/cm | |||
| Previous product Residue | No residue of previous product | |||
| Water for Injection | Before batch manufacturing | Description | Clear colourless free from particulate matter | QC/Micro |
| pH | 5.0 to 7.0 | |||
| Conductivity | NMT 1.3 µs/cm | |||
| BET | NMT 0.25 EU/ml | |||
| Bulk Mixing | From Manufacturing tank (After 05, 10, 15 minutes Mixing) Top + Bottom at 2880 RPM & Composite Bulk | Description | As per Specification | QC/Micro |
| pH | ||||
| Assay | ||||
| Bio Burdon | ||||
| Bulk sample before filtration (Covering Hold Time Study) | From Manufacturing tank Initial Zero Hour | Description | QC/Micro | |
| pH | ||||
| Assay | ||||
| Bio Burdon | ||||
| From Manufacturing tank After 24 Hours | Description | QC/Micro | ||
| pH | ||||
| Assay | ||||
| Bio Burdon | ||||
| From Manufacturing tank After 48 Hours | Description | QC/Micro | ||
| pH | ||||
| Assay | ||||
| Bio Burdon | ||||
| Bulk sample after filtration (Covering Hold Time Study) | From Filtration area Initial Zero Hour | Description | QC | |
| pH | ||||
| Assay | ||||
| Sterility | Micro | |||
| BET | ||||
| From Filtration tank After 24 Hours | Description | QC | ||
| pH | ||||
| Assay | ||||
| Sterility | Micro | |||
| From Filtration tank After 48 Hours | Description | QC | ||
| pH | ||||
| Assay | ||||
| Sterility | Micro | |||
| From Filtration tank After 72 Hours | Description | QC | ||
| pH | ||||
| Assay | ||||
| Sterility | Micro | |||
| BET | ||||
| Washing Ampoules | Washed Ampoule (For PV batches initial, Middle, End) | Clarity | QC | |
| Depyrogenated Ampoule | From Turn table in ampoule filling & sealing room (PV batches Initial, Middle, End of batch at the start of filling) | Sterility | Micro | |
| Composite Sample | BET | |||
| Filling & Sealing | Initial, Middle and End Stage of filling Duration | Description, pH, Assay, Fill Volume & Sterility | QC/Micro | |
| Finished sample | Finish Stage | Testing as per finished product Specification | QC/Micro | |
| Packaging of filled Ampoules | Initial, Middle and End Stage of Packaging Duration | Correctness of coding and packing | QA/Production |
12.4 SAMPLING LOCATIONS:
MIXING VESSEL:

T = Top Layer
B = Bottom Layer
T + B = Composite
13.0 REVALIDATION:
Revalidation shall be considered and carried out when any of the following conditions occur:
• Periodical Validation
• Change in critical formulation component i.e. raw material
• Change in manufacturer or vendor of Active Pharmaceutical Ingredient
• Change in critical specifications of the product
• Change in manufacturing process which may affect the quality of the products.
• Change in the facility and /or plant (location or site)
• Any major change in equipment.
Note: In case of the requirements for revalidation, because of above mentioned reasons, the validation of the critical steps
shall be undertaken through addendum protocol to this protocol or a separate protocol.
14.0 VALIDATION STABILITY PLAN:
• The three validation batches shall be subjected to stability studies as per stability plan.
15.0 REFERENCES:
The Principle References are as following:
• Relevant Specifications and Standard Testing Procedures
• Relevant Standard Operating Procedures
• Relevant Qualification Documents
• Indian Pharmacopoeia
• United State Pharmacopoeia
• Supplementary Guidelines on (GMP): Validation;
16.0 DOCUMENTS TO BE ATTACHED:
• Records for all critical parameters with graphical representation, where applicable.
• Relevant Sterilization Charts
• Raw Data of Validation Testing.
• Certificate of Analysis of API.
• Certificate of Analysis of Finished Product.
17.0 NON COMPLIANCE, IF ANY:
All the Non-compliances of procedure, specifications, and sampling, analysis and documentation activities shall be monitored & recorded.
18.0 DEVIATION FROM PRE-DEFINED SPECIFICATION, IF ANY:
• In case of any deviation observed during process validation, inform to Head QA for necessary action.
• Document the deviation detail in observed deviation section.
• The Head QA will study the impact of deviation. If deviation is acceptable and it does not have an impact on operation as well as on performance of the machine & prepare final conclusion.
19.0 CHANGE CONTROL, IF ANY:
• If any change control is required during process validation, inform to Head QA for necessary action.
• Document the details observed.
• The Head QA will study the impact of change. If change is acceptable and it does not have an impact on operation as well as on performance of the machine & prepare final conclusion.
20.0 ABBREVIATIONS:
PVP : Process Validation Protocol
mg : Milligram
KG : Kilogram
Ltr. : Liter
QS : Quantity Sufficient
BMR : Batch Manufacturing Record
BPR : Batch Packing Record
QA : Quality Assurance
QC : Quality Control
LOD : Loss on drying
% : Percentage
NLT : Not Less Than
NMT : Not More Than
SOP : Standard Operating Procedure
°C : Degree Centigrade
RPM : Rotation per minutes
WFI : Water For Injection
Kg/cm2 : Kilogram per centimeter square
DP : Differential pressure
cfu : Colony Forming Unit
EU/ml : Endotoxin unit per milliliter
WHO : World Health Organization
21.0 REVISION HISTORY:
| Revision No. | Change Control No. | Detail of Changes | Reason for Change | Effective Date | Updated By |
| 00 | NA | NA | New Protocol |
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipment
concurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
please provide the dry injection process validation protocol and report