sop for process validation
1.0. OBJECTIVE:
The objective of this SOP is:
1.1 To describe the procedure for process validation.
2.0. RESPONSIBILITY:
2.1 The Officer – Quality Assurance
2.1.1. Responsible to carry out the sampling as per sampling plan mentioned in validation protocol.
2.1.2. Responsible for sending intimation on sample withdrawal to Quality control for analysis.
2.2 The Officer – Quality Control
2.2.1 Responsible for analysing the samples as per specification / Validation Protocol.
2.2.2 Responsible for interpreting the results obtained and maintaining the raw data there of.
2.2.3 Responsible for getting Approvals of results from Head – quality control.
2.3 The Executive – Quality Assurance
2.3.1 Responsible for preparing the Process Validation Protocols.
2.3.2 Responsible for co-ordination with production and Quality Control to fulfil the Qualification and validation requirement
2.3.3 Responsible for final compilation and review of data and conclusion.
2.4 The Head – Research and Development shall be:
2.4.1 Responsible for change of formula / process / specification based on validation results, if required.
3.0. ACCOUNTABILITY:
Head – Quality Assurance
4.0. PROCEDURE:
4.1 All the processes either new product or site change shall be validated as per process given below;
4.2 Before commencing the Process Validation it shall be ensured that, all the equipments, utilities, analytical systems etc.
have been qualified (Installation Qualification, Operational Qualification and performance Qualification) as per SOP
4.3 Process validation shall be carried out for any new product at least three batches. Product shall not be released for distribution until the process validation completed.
4.4 Process validation for new product shall be done as per the Master Process Validation Protocol – New product. Validation protocol shall include the following details;
a. Short Description of the process.
b. Summary of the critical processing steps with operational parameters to be investigated (Experimental plan and limits).
c. List of the equipment / facilities to be used (including measuring / monitoring / recording equipment) together with its calibration status.
d. Finished product specifications for release.
e. List of analytical methods, as appropriate.
f. Proposed in-process controls with acceptance criteria.
g. Additional testing shall be carried out (if any), with acceptance criteria and analytical validation, as per requirement and necessity.
h. Sampling plan
i. Stability Study
4.4.1 The number of process runs carried out and observations made shall be sufficient to allow the normal extent of variation and trends
to be established and to provide sufficient data for evaluation
4.4.2 Batches made for process validation shall be of the same size as per the intended scale up batches.
4.5 Analytical testing methods used for validation shall be validated. Analyst taking part in the validation work shall have been appropriately trained.
4.6 Site Change: If the product is already launched and manufactured at other locations and it is shifted to Amaliya plant then carry out the process
optimisation as per the guideline mentioned in Master Process Validation Protocol– Site Change and make the product specific protocol
with less rigorous monitoring of analytical parameters.
4.7 Retrospective Validation:
4.7.1 Validation Processes in use for some time shall also be validated (Retrospective validation) for old products, which have been performed at other locations.
4.7.2 Validation of such products shall be based on historical data. The steps involved require the preparation of a specific protocol and the reporting
of the results of the data review, leading to a conclusion and a recommendation.
4.7.3 The source of data for batch shall include, but not limited to batch manufacturing records, process control charts, maintenance logbooks,
process capability studies, finished product data, including trend charts and storage stability results.
4.7.4 Batches selected for retrospective validation shall be a representative of all batches made during the review period, including any
batches that failed to meet specifications, and should be sufficient in number to demonstrate process consistency.
4.7.5 Atleast 20 no. of batches shall be subjected to retrospective validation.
4.7.6 Product specific protocol shall be prepared for each product for retrospective validation.
4.8 Re-Validation.
4.8.1 The criteria for revalidation is given below;
a. When there is a change in manufacturing site, the process optimisation shall be carried out in two batches with less rigorous monitoring.
b. When there is change in formulation or Critical Process Parameters.
c. Change in Batch Size.
d. Change of critical Equipment / critical part of equipment, which is directly affecting the final Quality of the product.
e. Change of Approved vendor source of Active Pharmaceutical Ingredient (API).
4.9 Methodology To Be Followed For Process Validation:
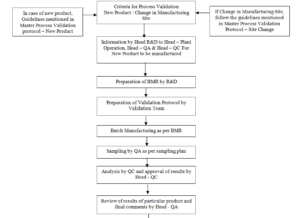
4.9.1 In case of any new product, the Research & Development department shall communicate to the Head – Plant Operation,
Head – Quality Assurance and Head – Quality Control regarding the same.
4.9.2 Head – R&D prepares a tentative Batch Manufacturing Record.
4.9.3 Validation team shall prepare Process Validation protocol incorporating validation procedure for each critical step
inclusive of the environmental conditions prevailing and specifying the acceptance criteria for each step on the basis of R & D’s BMR.
4.9.4 The process validation protocol shall include the following;
a. Process Flow.
b. The process steps to be monitored.
c. Process critical parameters to be monitored and their limits.
d. Sampling schedule and sampling plan.
e. Statistical evaluation tool to be used to analyse the data.
f. Finished Products specifications and their limits (Acceptance criteria)
4.9.5 The Production department then under takes the manufacturing of the batch in co-ordination with the Validation team.
4.9.6 The required samples are withdrawn by the Quality Assurance for each critical step & forwarded to the Quality Control department.
4.9.7 The samples shall be analysed by the Quality Control & the results shall be forwarded to the Validation Team on approval of Head – Quality Control.
4.9.8 Statistical evaluation on completion of validation shall be done.
4.9.9 If the results meet the required acceptance criteria for all the steps for three consecutive batches of a particular Product, after review
of Batch manufacturing Process by Head – Quality Assurance, the same shall be approved.
4.10 Numbering System for Process Validation Protocol:

5.0 TRAINING:
Trainer — Head – Quality Assurance
Trainees — Validation team, Quality Assurance / Quality Control / Production Personnel
6.0 DISTRIBUTION:
Certified Copy : Head of Department – Quality Control
Certified Copy : Head of Department
Certified Copy : Head – R & D
Certified Copy : Head – Plant Operation.
Original Copy : Head – quality assurance.
7.0 ANNEXURES:
Annexure – 1 : Schematic Diagram for Process Validation.
8.0 REFERENCES:
In house
ANNEXURE – 1
SCHEMATIC DIAGRAM FOR PROCESS VALIDATION

process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipment
concurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection