sop for Pressure Differential Monitoring
1.0 Objective
To lay down a procedure for Pressure Differential Monitoring.
2.0 Scope
This Standard Operating Procedure is applicable for recording of Differential
Pressure between tow adjacent Rooms to be followed at formulation plants of abc Pvt. Ltd.
3.0 Responsibility
3.1 Officer / Executive of concerned department shall be responsible for monitoring the differential
pressure and note its observations in respective documents.
3.2 Officer / Executive QA shall be responsible to verify differential pressure in the respective area.
3.3 Concerned department head shall be responsible for implementation of this SOP.
3.4 Head QA shall be responsible for compliance of this SOP.
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
QA : Quality Assurance
No. : Number
HVAC : Heating, ventilation and air conditioning
5.0 Procedure
5.1 Ensure that both the HVAC and Dust extraction system (where ever applicable) are in working condition.
5.2 Avoid movement of personnel in the area at the time of observation of differential pressure.
Ensure that all the doors in the areas are in closed condition.
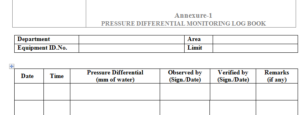
5.3 Take the readings room wise and note down in the Annexure-1. The reading in the gauge it self
shows the differential pressure between the high pressure area and low pressure area in mm of water.
5.4 In case the readings are out of limits take following action:
5.4.1 Clean the room returns filters.
5.4.2 Take fresh readings for that room. If the problem persists than contact the Engineering department for further action.
5.4.3 If problem still persists, suspend all activity where product is exposed to environmental till
the problem is rectified. Operations under closed system may be continued.
5.4.4 Keep all the doors closed to avoid cross contamination.
5.5 Frequency: twice in a shift.
6.0 Forms and Records
6.1 Pressure Differential Monitoring Record – Annexure-1
7.0 Distribution
7.1 Master copy – Documentation Cell (Quality Assurance)
7.2 Controlled copies – Production, Engineering and Warehouse
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |
- process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
-
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
sop for Verification of Weighing Balance
-
Sop for Submitting of Leave Application
sop for Accident Management Procedures
sop for Accident Management Procedures
Cleaning and colour coding of Factory Apparel (Clothes)
Job Responsibilities of Key Personnel
sop for Maintenance of Building
Destruction of Batch Production and Control Records BPCR