Measurement and Recording of Temperature and Relative Humidity
1.0 Objective
To lay down a procedure for measurement and recording of temperature and relative humidity.
2.0 Scope
This Standard Operating Procedure is applicable for measurement and recording of
temperature and relative humidity to be followed at formulation plants of ABC Pvt. Ltd.
3.0 Responsibility
3.1 Officer / Executive of concerned department shall be responsible for measurement and
recording of temperature and relative humidity.
3.2 Concerned department head shall be responsible for implementation of this SOP.
3.3 Head QA shall be responsible for compliance of this SOP.
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
BPCR : Batch Production and Control Record
FG : Finished Goods
RH : Relative Humidity
QA : Quality Assurance
No. : Number
5.0 Procedure
5.1 Measurement of temperature and relative humidity with wet and dry thermo hygrometer
5.1.1 Officer / Executive of concerned department shall ensure that the thermo hygrometer is
calibrated and status tag of calibration is affixed on hygrometer.
5.1.2 Before monitoring the relative humidity, Officer / Executive shall ensure that the water
reservoir is filled with purified water so that wick remains wet.
5.1.3 Empty out the water reservoir every week and clean it properly.
5.1.4 After refilling with purified water in reservoir ensure that wick has wet completely and temperature gets stabilized.
5.1.5 Officer / Executive shall ensure that the wick is properly inserted in reservoir of thermo hygrometer.
5.1.6 Place the dry bulb/ wet bulb hygrometer in the area where temperature and relative humidity
to be monitor, keep the Psychometric chart and monitoring record near the hygrometer or in area.
5.2 Monitor the relative humidity as per the procedure given below
5.2.1 Read the dry bulb temperature and wet bulb temperature in °C visually by seeing
the mercury level of the thermometer.
5.2.2 Calculate the difference between dry bulb and wet bulb temperature.
5.2.3 See the deference against dry bulb temperature in psychometric chart and read the relative humidity.
5.3 Monitoring of temperature and relative humidity by using digital thermo hygrometer
5.3.1 Officer / Executive shall ensure that the digital thermo hygrometer is duly calibrated
and status tag of calibration is affixed on digital thermo hygrometer.
5.3.2 Place the digital thermo hygrometer in the area where temperature and humidity record is to be monitor.
5.3.3 Officer / Executive shall ensure that thermo hygrometer shall be placed in a location where
there is no disturbance from machine vibration, water spillage or any other disturbances.
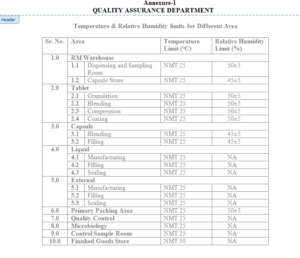
5.4 Refer the temperature and relative humidity limit for specified location as mentioned in
Annexure-1 and record the observation in Annexure-2 for area where the wet thermo
hygrometer is placed, refer Annexure-3 for recording where digital thermo hygrometer is used
and refer Annexure-4 & Annexure-5 for recording the temperature of area where the relative
humidity not applicable there shall be the remain ambient humidity.
5.5 Monitor the temperature and relative humidity of the area at the frequency of twice in a
shift, i.e. at the start of the shift and at the end of the shift and record the same in
format as shown in Annexure-2 to Annexure-5.
5.6 In case a product specific, temperature & relative humidity are mentioned in BPCR,
the same shall be maintained and recorded in BPCR.
5.7 Temperature shall be read visually by observing the mercury level of the
dry bulb thermometer avoiding parallax error.
5.8 In case if temperature and relative humidity observed is above or below specified
limit mentioned in Annexure-1, stop the activity in respective area immediately and
inform the concern head, Engineering Department for necessary rectification
and also inform to Head QA or his / her designee.
5.9 In the remarks column mention the reason (if any) for not entering the temperature
and relative humidity or for any abnormal reading.
6.0 Forms and Records
6.1 Temperature & Relative Humidity limit for different area – Annexure-1
6.2 Environmental Monitoring Record (wet & dry) – Annexure-2
6.3 Environmental Monitoring Record (Digital) – Annexure-3
6.4 Environmental Monitoring Record (Dry) – Annexure-4
6.5 Environmental Monitoring Record – Annexure-5
7.0 Distribution
7.1 Master copy – Documentation Cell (Quality Assurance)
7.2 Controlled copies – Production, Quality Assurance, Quality Control, Engineering and Warehouse.
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |




- process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
-
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
sop for Verification of Weighing Balance
-
Sop for Submitting of Leave Application
sop for Accident Management Procedures
sop for Accident Management Procedures
Cleaning and colour coding of Factory Apparel (Clothes)
Job Responsibilities of Key Personnel
sop for Maintenance of Building
Destruction of Batch Production and Control Records BPCR
Procedure for Sampling of Rinse water Swab
Cleaning Validation of Equipment
Measurement and Recording of Temperature and Relative Humidity