procedure for cleaning validation
1.0. OBJECTIVE:
The objective of this SOP is:
1.1 To describe a procedure for cleaning validation
2.0. RESPONSIBILITY:
2.1 The Officer – Quality Assurance shall be:
2.1.1. Responsible for carry out the sampling as per sampling plan mentioned in protocol.
2.1.2. Responsible for sending intimation to Quality control for analysis.
2.2 The Officer – Quality Control shall be:
2.2.1 Responsible for analysing the samples as per specification.
2.2.2 Responsible for interpreting the results obtained and maintaining the raw data there of.
2.2.3 Responsible for getting Approvals of results from Head – Quality Control.
2.3 The Executive – Quality Assurance shall be:
2.3.1 Responsible for preparing the Cleaning validation protocols by considering the guidelines given in SOP.
2.3.2 Responsible for taking approvals of respective persons mentioned in protocol.
2.3.3 Responsible for co-ordination with Production and Quality Control to full fill the validation of cleaning procedure.
2.3.4 Responsible for final compilation and review of data.
2.4 The Head – Quality Assurance shall be:
2.4.1 Responsible for approval of protocols before commencing the validation activities.
2.4.2 Responsible for monitoring entire activity of validation.
2.4.3 Responsible for review and approval of validation data.
3.0. ACCOUNTABILITY:
Head – Quality Assurance
4.0. PROCEDURE:
4.1 Basic Elements Of Cleaning Validation
4.1.1 Activities that shall be performed during cleaning validation depend on the nature of the equipment and facility, as well as on the type of products manufactured on the equipment or facility.
4.1.2 During the preparation of cleaning procedure and its validation, and especially during determination of acceptability limits, the following issues shall be taken in consideration:
• Purpose of equipment
• Equipment design
• Type of contaminant
• Assessment of GMP relevance
4.2 Types Of Contaminants
4.2.1 The selection of the method of cleaning depends primarily on the characteristic of contaminant or material that have to be removed from the equipment during cleaning process.
4.2.2 Residual contaminants after cleaning may be:
• Chemical – residues of the previous product.
• Biological – microorganisms
• Physical – particulate matter.
4.3 Qualification Of The Cleaning Procedure
4.3.1 Cleaning validation is performed according to the plan and activities described in the Qualification Protocol. In order to initiate cleaning validation, the following prerequisites should be met:
• Defined, published cleaning procedures, approved by Quality Assurance
• Defined and validated methods of analysis
• Defined sampling
• Trained employees.
4.4 Cleaning Procedures:
4.4.1 For each equipment there shall be a defined and detained SOP, which states the cleaning agents, accessories to be used and cleaning procedures.
4.4.2 After the cleaning, tests are performed to confirm the efficacy of cleaning of individual parts of the equipment, however they cannot substitute cleaning validation which proves the efficacy of cleaning of entire systems or production lines.
4.4.3 The following factors should be taken into consideration when defining cleaning procedures:
• Physical and chemical characteristics of contaminants (solubility, etc.,)
• Cleaning agents (solvents, detergents, etc.,)
• Compatibility of cleaning agents with the material from which the equipment is made.
• Volumes of used cleaning and rinsing agents (water, solvents, etc.,)
• Rinsing time (under running water where possible)
• Quality of utilities (water for washing and rinsing, air for drying, etc.,)
• Temperature
• Cleaning sections and the sequences of operations.
• Time of draining and drying of equipment
• Duration and frequency of cleaning
• Interval between cleaning and the use of the equipment
• Identification of the equipment which is cleaned
• Status of the equipment (clean, unclean, etc.,)
• Trained and responsible personnel for implementation of cleaning procedures.
4.4.4 Cleaning instructions should be approved by responsible persons from Production and Quality Assurance.
4.5 Sampling:
4.5.1 Sampling methods which will be used during validation of cleaning of production equipment must be selected in advance and stated in the protocol of qualification of processes, as well as the surface and/or volume of the taken sample.
4.5.2 It is best to combine swabs and rinses, although each of them is appropriate as a single sampling method. For microbiological testing, swab is taken from places estimated to be theons with the highest possibility of retention of microorganisms after washing.
4.5.3 Cleaning Validation Protocol includes the type of accessories and packaging, sampling procedure and sampling plan and labeling of samples. For calculation of contaminant residues in the process, the information about the recovery from the swab and/or rinse is required.
4.6 Methods of analysis:
4.6.1 Methods of analysis used for determination of possible contaminant residues must be specific and sensitive.
4.6.2 Parameters to be taken into consideration in selection of the method of analysis are detection limit, quantification limit, specificity and accuracy. This is described in more detail in SOP No:
4.6.3 When the calculated acceptability limit can be modified or another method of analysis used. The modified acceptability limit must be documented and scientifically proven.
4.6.4 The methods of analysis used during cleaning validation must also be validated and stated in the process qualification protocol.
4.6.5 The method of analysis and sampling method must be combined to enable as efficient as possible removal of contaminant from the surface of the equipment and the level of recovery with which this is achieved should be determined. This should be done before making any conclusions based on results of analysis of samples, since negative result may also arise due to poorly selected sampling technique (Spike Recovery).
4.7 Acceptability limits:
4.7.1 During the preparation of the cleaning validation protocol, the most important issue is to determine the acceptability limit.
4.7.2 During determination of the acceptability limit for cleaning validation, the calculation of acceptable level of the previous product as contaminant is of special importance.
4.7.3 Validation protocol must include the list of all products manufactured on the equipment for which cleaning validation is performed.
4.7.4 The following data should be included for all products:
Contact surface of non-dedicated equipment
Batch size
Number and size of unit packages
Minimum daily therapeutic dose of the next product
Maximum daily therapeutic dose of the next product.
Surface or volume of the sample
Level of recovery of the swab or rinse, level of recovery from the surfaces of the equipment.
Permitted amount of contaminant in the sample.
4.7.5 Two basic acceptability limits are recommended.
4.7.5.1 Visually clean
Clean to Eye
4.7.5.2 1/1000of minimum daily therapeutic dose
4.7.5.2.1 Not more than 1/1000 of minimum daily therapeutic dose of the previous product in the maximum daily dose of the product, calculated with respect to the total weight of the dosage form in the maximum daily therapeutic dose.
4.8 Calculation of Contaminant Residue.
4.8.1 Maximum Allowable carry over of previous product in the subsequent product has been calculated by formula mentioned below,
— MAC = TD X BS X SF
— LDD
4.8.2 Where: MAC: the maximum allowable carry over; TD: a single therapeutic dose; BS: the batch size of the next product to be manufactured in the same equipment; SF : the safety factor; LDD: the largest daily dose of the next product to be manufactured in the same equipment
4.8.3 Sampling technique: Two methods of sampling are considered; Swab sampling and Rinse Water Sampling for routine line clearance.
4.8.4 Analytical method validation has been carried out for: Specificity, Precision, Linearity, Limit of detection (LOD), Limit of Quantitation (LOQ), System suitability at very low concentration.
4.8.5 When the process consists of several operations, cumulative effect of all individual operations must be taken into account for calculation of acceptability limits.
4.8.6 The calculation is based on the uniform distribution of contaminants in the process. If the process is a complex one consisting of several operations, the sum of the calculated values for the contaminant residues for individual parts of the equipment must not exceed the acceptability limit. Contamination residues for all non-dedicated equipment must be calculated.
4.9 DOCUMENTATION
4.9.1 The basic documentation on cleaning validation includes Cleaning Validation Protocol and Cleaning Validation Report.
4.9.2 Cleaning Validation Protocol is a documented plan, based on the principal validation plan, and is include in it.
4.9.3 Cleaning Validation Report is a document that must be in accordance with the Process Qualification Protocol, describing validation activities, summing and evaluating results of analysis. In the conclusion, it states whether the used cleaning procedures results in meeting the set acceptability limits and can be considered as validated.
4.9.4 Cleaning Validation documentation also includes Cleaning Procedures, Methods of Analysis with accompanying Validation and Sampling Methods.
4.10 CHANGE CONTROL:
4.10.1 All aspects of cleaning should be included in the change control system in order to obtain an overview of the proposed or implemented changes, which includes determining whether a corrective action will have impact on the rest of the system and validation status.
4.10.2 Cleaning procedures, methods of analysis, equipment, cleaning agents, and product formulations must be documented before the validation. Changes of procedures or documents must be documented and approved by the responsible and authorized person.
4.10.3 When the change is of such a nature as to significantly change the philosophy of the validation of cleaning, re-validation should be performed. Re-validation is the repeating of the initial validation with the objective to ensure that the changes will not have impact on the quality of products and production.
4.11 RE-VALIDATION:
4.11.1 After completion of the initial validation of cleaning procedure, the assessment of the requirement for re-validation is performed, which must be defined in the Cleaning Validation Report.
4.11.2 Cleaning re-validation must be performed during introduction of a new plant, facility, equipment, process or product, as well as when current cleaning procedure is changed (e.g., change of the cleaning agent) or after a pre-defined time period. Manual washing requires more frequent re-validation than automatic washing. It can be recommended that only parts of the initial cleaning validation plan are repeated during re-validation. There can also be changes in the method of sampling and the number of samples taken.
5.0. REASON FOR REVISION:
New SOP.
6.0 TRAINING:
Trainer — Head – Quality Assurance
Trainees — Validation team, Quality Assurance / Quality Control / Production / Warehouse Personnel
Period — One day
7.0 DISTRIBUTION:
Certified Copy No. 1 : Head of Department – Quality Control (Chemical)
Certified Copy No. 2 : Head of Department – Quality Control (Microbiology)
Certified Copy No. 3 : Head of Department – C- Block
Certified Copy No. 4 : Head of Department – General Block
Certified Copy No. 5 : Head – Warehouse
Certified Copy No. 6 : Head – Engineering
Certified Copy No. 7 : Head – Plant Operation
Original Copy : Head – QUALITY ASSURANCE.
8.0 ANNEXURES:
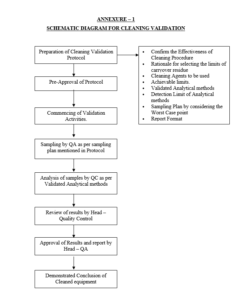
Annexure – 1 : Schematic Diagram for Cleaning Validation.
9.0 REFERENCES:
In house.
Annexure – 1 : Schematic Diagram for Cleaning Validation.
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipment
concurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection