cleaning validation protocol capsule manufacturing equipment
| Scope of validation | Capsule Manufacturing Equipment |
| Document No. | |
| Supersede No. | |
| Effective Date |
1.0 PROTOCOL APPROVAL:
1.1 This protocol is prepared by
| Department | Quality Assurance |
| Signature
|
|
| Name | |
| Designation | |
| Date |
1.2 This protocol is reviewed by
| Department | Quality Assurance | Quality Control | Production | Engineering |
| Signature
|
||||
| Name | ||||
| Designation | ||||
| Date |
1.3 This protocol is authorized by
| Signature
|
|
| Name | |
| Designation | |
| Date |
2.0 TABLE OF CONTENT:
| SR.
NO. |
CONTENT | PAGE
NO. |
| 1.0 | PROTOCOL APPROVAL | |
| 2.0 | ||
| 3.0 | OBJECTIVE | |
| 4.0 | SCOPE | |
| 5.0 | RESPONSIBILITIES | |
| 6.0 | INTRODUCTION OF CLEANING VALIDATION | |
| 7.0 | CLEANING PROCESS DESCRIPTION | |
| 7.1 | Brief description of the manufacturing process and processing equipment | |
| 7.2 | Review of cleaning document and cleaning procedure | |
| 7.3 | Equipment evaluation data for Seleact product | |
| 7.4 | Selection of worst case product for cleaning validation study | |
| 8.0 | METHODOLOGY OF CLEANING PROCESS VALIDATION | |
| 8.1 | Sampling plan & Sampling Location | |
| 8.2 | Acceptance Criteria | |
| 8.3 | Maximum allowable carryover | |
| 8.4 | Recovery study of stainless steel | |
| 8.5 | Analytical Method Validation (Laboratory procedures and its rationale) | |
| 8.6 | Sampling Technique | |
| 8.7 | Swab sampling and analysis technique | |
| 8.8 | Rinse sampling and analysis technique | |
| 8.9 | Visual Inspection technique | |
| 8.10 | Un-Cleaned equipment hold time | |
| 8.11 | Cleaned equipment hold time | |
| 10.0 | FAILURE INVESTIGATION AND CORRECTIVE ACTION | |
| 11.0 | DOCUMENTATION | |
| 12.0 | REVALIDATION | |
| 13.0 | CONCLUSION | |
| 14.0 | REFERENCE |
3.0 OBJECTIVE:
The objective of this protocol is to define approach to validation of cleaning procedures for Capsule manufacturing, and Packing . In addition to assure that there is no risk associated with cross-contamination of active ingredients.
4.0 SCOPE:
This protocol is applicable to validate the cleaning procedure of equipment used for the manufacturing and Packing of Tablet.
5.0 RESPONSIBILITIES:
5.1 Quality Assurance department shall be responsible for
5.1.1 Preparation of protocol.
5.1.2 Scheduling & conducting of validation
5.1.3 Monitoring of protocol completeness, accuracy, technical excellence and applicability
5.1.4 Data compilation and review the data/report
5.1.5 Validation reports preparation and recommendation thereafter (if required)
5.1.6 Approval of validation protocol
5.2 Production department shall be responsible for
5.2.1 To provide all applicable cleaning and operational procedures and documentation necessary for the generation of this protocol
5.2.2 To release equipment and accessories for validation
5.2.3 To provide personnel to assist in the preparation and execution of this protocol
5.2.4 Review the protocol and report.
5.3 Quality Control department shall be responsible for
5.3.1 To provide all applicable methodologies, analytical procedures and documentation necessary for generation and execution of this protocol.
5.3.2 All collected samples received from QA and analysis as per sampling plan.
5.3.3 To compile the analytical data.
5.3.4 To provide personnel to assist in the preparation and execution of this protocol.
5.3.5 Review the protocol and report.
5.4 Engineering department shall be responsible for
5.4.1 To provide all equipment contact surface area.
5.4.2 To provide the utilities for CIP and manual cleaning.
5.4.3 Review the protocol and report.
6.0 INTRODUCTION OF CLEANING VALIDATION:
This cleaning validation is to verify the effectiveness and consistency of the cleaning procedure for removal of product residues, preservatives, Excipients and/or cleaning agents as well as the control of potential microbial contaminants in product Selection of “Worst case” product on the basis of toxicity, solubility and Pharmacological action (Potency) of Active pharmaceutical Ingredients (API). if required the grouping approach for the equipment can be considered in the cleaning validation. Three consecutive applications of the cleaning procedure shall be performed during cleaning validation to justified effectiveness & reproducibility of the cleaning.
If any product introduced or discontinue into the line then revise the product matrix and perform cleaning validation accordingly if get any change in ‘Worst case’.
7.0 CLEANING PROCESS DESCRIPTION
7.1 Brief description of the cleaning process and processing equipment :
Manufacturing:- As per SOP
Compression:- As per SOP.
Coating:- As per SOP .
Packing:- followed by carton and corrugated Box Pack.
7.1.1 Process equipment : The equipment used in the processing and with direct contact to the
product
7.2 Review of Cleaning Documents & Cleaning procedure :
7.2.1 List of equipment to be cleaned & rationale :
This protocol will address the cleaning of the following product contact equipment used to manufacture the Capsule
| Sr. No. | Sop No. | Title | Effective date | Equipment | Criticality Rating |
| 1 | Operation & Cleaning Procedure of Vibro Sifter | Vibro Sifter | Critical | ||
| 2 | Operation & Cleaning Procedure of Octagonal Blender | Octagonal Blender | Critical | ||
| 3 | Operation & Cleaning Procedure of Automatic Capsule Filling Machine | Automatic Capsule Filling Machine | Critical | ||
| 4 | Operation & Cleaning Procedure of Blister Packing Machine | Blister Packing Machine | Critical |
7.2.2 List of contact parts which are directly contact with the product during manufacturing.
| Sr. No. | Equipment Name | Equipment ID | Contact parts |
| 1 | Vibro Sifter | Lid & Sieve | |
| 2 | Octagonal Blender | Lid & blender | |
| 3 | Automatic Capsule Filling Machine | Hopper, Plate & Chute | |
| 4 | Blister Packing Machine | Hopper & Chute |
7.2.3 Cleaning Procedure:
7.2.3.1 Manual Cleaning Process:
The cleaning procedure as per reference SOP No. given in point 7.2.1 provides details of the procedure ,equipment and material; is required in order to conduct manual cleaning of the product manufacturing process equipments.
7.2.4 Holding Times:
| Sr. No. | Equipment name | Un cleaned Equipment Hold time | Cleaned Equipment hold time |
| 1 | Vibro Sifter | Minimum 30 hr | Minimum 72 hr |
| 2 | Octagonal Blender | Minimum 30 hr | Minimum 72 hr |
| 3 | Automatic Capsule Filling Machine | Minimum 30 hr | Minimum 72 hr |
| 4 | Blister Packing Machine | Minimum 30 hr | Minimum 72 hr |
7.3 Equipment evaluation (Surface area) data:
| Sr. No. | Equipment | Make | Equipment ID | Material of Construction | Surface area
(square inch) |
| 1 | Vibro Sifter | Techx Process Automation | SS 316 | ||
| 2 | Octagonal Blender | Techx Process Automation | SS 316 | ||
| 3 | Automatic Capsule Filling Machine | Anchor Mark | SS 316 | ||
| 4 | Blister Packing Machine | Techx Process Automation | SS 316 | ||
| Total | |||||
7.4 Selection of worst case product for cleaning validation study
| SR.NO | PRODUCT NAME | GENERIC NAME | ACTIVE PHARMACEUTICAL INGREDIENT (API) | Potency mg per tablet
|
Solubility in water | Toxicity (mg/kg) | B. Size
(Tablets)
|
| 1 | Chloramphenicol Capsule | Each hard gelatin capsule contains: Chloramphenicol USP 250 mg | Chloramphenicol 250 mg | Insoluble | 3,50000 | ||
| 2 | Rabeprazole Sodium & Domperidone Capsule |
Each hard gelatin capsule Contains: Rabeprazole Sodium 20 mg (As enteric Coated Granules) Domperidone BP 30 mg (As sustained release granules) |
Rabeprazole Sodium 20 mg Domperidone 30 mg |
Insoluble
Practically Insoluble |
|
1,00000 | |
| 3 | Oxetetracycline Hydrochloride Capsule | Each Capsule Contains: Oxetetracycline Hydrochloride IP 250 mg |
Oxetetracycline Hydrochloride 250gm | Soluble | 1,00000 | ||
| 4 | Pantoprazole Sodium Sesquihydrate & Domperidone Maleate Capsule |
Each Hard gelatin capsule Contains: Pantoprazole Sodium Sesquihydrate IP Eq. to Pantoprazole 40 mg (As enteric Coated Granules) Domperidone Maleate IP eq to Domperidone 30 mg (AS sustained release Granules) |
Pantoprazole 40 mg Domperidone 30mg |
Soluble
Practically Insoluble |
|
3,00000 |
Note:- 1) Based on the above data Capsule is become as “Worst Case” product hence it is considered in Cleaning Validation.
8.0 METHODOLOGY OF CLEANING PROCESS VALIDATION
When the process is completed then note the time and date step wise. Record the start of un cleaned hold time, after 30 hrs note the time with date for evaluation of un-cleaned hold time.
After 30 hr take swab and rinse sample for microbial evaluation as per respective SOP
Take the sample for un-cleaned hold time as per sampling plan given at page no. 30.
Clean all equipments as per respective SOP for all equipment given at point no. 7.2
Initially take the microbial swab as point no. 8.7 then Take rinse sample for chemical analysis as per point no. 8.8
After collection of samples hold all equipments and accessories for 72 hrs. After 72 hrs again collect the samples of all equipments and accessories as per sampling plan . After 72 hr. note the time and date as per sampling plan for evaluation of cleaned equipment hold time. First take swab sample for microbiology, then chemical rinse. Send these samples to QC for analysis.
Sample shall be taken from hardest to clean surface of the equipment(i.e Dead legs, Dead spots).
Continue the above procedure for another two batches.
8.1 SAMPLING PLAN AND SAMPLING LOCATION FOR MICROBIAL TESTING (HOLD TIME)
| Sr. No. | Equipment | Sample Type | Sample Location | Sample Quantity
required |
Sample ID | Test Parameter | Acceptance Criteria |
| 1 | Vibro Sifter
|
Swab | Granulation | NA | Total Aerobic Count | ||
| Bacteria | NMT100 cfu | ||||||
| Fungi | Nil | ||||||
| 2 | Octagonal Blender
|
Swab | Blending | NA | Total Aerobic Count | ||
| Bacteria | NMT100 cfu | ||||||
| Fungi | Nil | ||||||
| 3 | Capsule Filling Machine | Swab | Filling | NA | Total Aerobic Count | ||
| Bacteria | NMT100 cfu | ||||||
| Fungi | Nil | ||||||
| 4 | Blister Packing Machine | Swab | Packing | NA | Total Aerobic Count | ||
| Bacteria | NMT100 cfu | ||||||
| Fungi | Nil | ||||||
8.1.1 SAMPLING PLAN AND SAMPLING LOCATION FOR CHEMICAL TESTING CLEANED EUIPMENT
| Sr. No. | Equipment | Sample Type | Sample Location | Sample Quantity
required |
Sample ID | Test Parameter | Acceptance Criteria |
| 1 | Vibro Sifter
|
Rinse | Granulation | 100ml | pH | 5.0-7.5 | |
| Conductivity | NMT 5.1 µS | ||||||
| TOC | NMT 500ppb | ||||||
| 2 | Octagonal Blender
|
Rinse | Blending | 100ml | pH | 5.0-7.5 | |
| Conductivity | NMT 5.1 µS | ||||||
| TOC | NMT 500ppb | ||||||
| 3 | Capsule Filling Machine | Swab | Filling | 100ml |
|
pH | 5.0-7.5 |
| Conductivity | NMT 5.1 µS | ||||||
| TOC | NMT 500ppb | ||||||
| 4 | Blister Packing Machine | Swab | Packing | 100ml | pH | 5.0-7.5 | |
| Conductivity | NMT 5.1 µS | ||||||
| TOC | NMT 500ppm |
8.1.2 SAMPLING PLAN AND SAMPLING LOCATION FOR MICROBIAL TESTING CLEANED EQUIPMENT
| Sr. No. | Equipment | Sample Type | Sample Location | Sample Quantity
required |
Sample ID | Test Parameter | Acceptance Criteria | |
| 1 | Vibro Sifter
|
Swab | Granulation | Total Aerobic Count | ||||
| Bacteria | NMT100 cfu | |||||||
| Fungi | Nil | |||||||
| 2 | Octagonal Blender
|
Swab | Blending | Total Aerobic Count | ||||
| Bacteria | NMT100 cfu | |||||||
| Fungi | Nil | |||||||
| 3 | Capsule Filling Machine | Swab | Filling | Total Aerobic Count | ||||
| Bacteria | NMT100 cfu | |||||||
| Fungi | Nil | |||||||
| 4 | Blister Packing Machine | Swab | Packing | Total Aerobic Count | ||||
| Bacteria | NMT100 cfu | |||||||
| Fungi | Nil | |||||||
8.2 ACCEPTANCE CRITERIA :
8.2.1 For cleaned equipment
For Chemical:
No more than 0.1% of the normal therapeutic dose of any product will appear in the maximum daily dose of the following product.
No more than 10 ppm of any product will appear in another product.
No quantity of residue should be visible on the equipment after cleaning procedures are performed. Spiking studies should determine the concentration at which most active ingredients are visible.
For microbial:
NMT100cfu / ml for rinse sample and NMT 100cfu /swab for swab sample.
8.2.2 For un-cleaned equipment
For microbial: For Information for rinse sample and swab sample.
8.3 Maximum Allowable Carryover:
Cleaning Limits Selection Criteria based on Maximum Allowable Carryover (MAC) :
8.3.1 MAC Based on 0.1 % Safety Factor
MAC = Lowest daily dose (mg) x Lowest B.Size (Kg) x 1000000
Largest Daily Dose (mg) x 1000
= 250 x112.203 x1000000 = 7012.6875mg
4000 x 1000
8.3.2 MAC based on 10 ppm = 0.00001mg
= 10 ppm x B.Size
= 0.00001 x112.203 x 1000000 = 1122 mg
Based on above criteria mg is the lowest value, hence it is consider as acceptance criteria.
8.3.3 MAC for each square inch.
MAC value = 7012 = 0.102 = mg/ square inch
Total surface area 68900.876
(Equipments)
8.3.4 MAC for swab sample.
Surface area for swab sample 5”x 5” .
Total surface area in swab sample = 5 x 5= 25 square inch.
MAC for each swab = MAC for each square inch x total surface area of swab
= 0.102 x 25 = 2.54 mg/swab
MAC for each swab = 2.54 mg/swab = NMT 254µg/swab
8.3.5 MAC for rinse sample of each equipment.
| Sr. No. | Equipment | MAC/square inch (mg) | Surface area
(square inch) |
MAC (mg) | MAC (µg) |
| 1 | Vibro Sifter | ||||
| 2 | Octagonal Blender | ||||
| 3 | Capsule Filling Machine | ||||
| 4 | Blister Packing Machine |
8. 4 Recovery study of Stainless Steel :
8.4.1 Perform analytical method validation and swab recovery studies. Spiking Studies for determining the concentration at which most active pharmaceutical ingredients.
8.4.2 Report the results applying the recovery factor for active pharmaceutical ingredients with consideration also taken for any chemical variants (active decomposition materials), write summary and conclusion. Record the recovery study .
8.4.3 ACCEPTANCE CRITERIA: Shall be Not Less than 80%.
8.5 Analytical Method Validation (Laboratory procedures and its rationale)
8.5.1 Analytical method to be used for cleaning validation shall be specific (i.e. either or combination of HPLC, TLC,) and validated for following parameters:
Specificity
Linearity
Limit of detection
Limit of quantification
8.5.2 Attach analytical method validation report .
8.6 Sampling technique :
There are two sampling technique :
8.6.1 Swab sample technique : This is direct surface technique use wet swab.
8.6.2 Rinse sample technique : This Technique is applicable where difficult to carry swab sample.
8.6.3 For uncleaned/cleaned equipment sample detail:
For microbial analysis: Purified water shall be use for microbial evaluation.
For chemical analysis : Purified water shall be used for swab & rinse sample.
8.7 Swab sampling and analysis technique
8.7.1 Take swab as per SOP NO
8.7.2 Select 4 to 5 square inch area of the surface and swab it by sterile cotton swab by dipping it in Peptone water tube test tube containing 2 ml Peptone water.
8.7.3 After swabbing , dip the swab in same peptone tube and shake it properly so as that it liberate adhered microbes into the Peptone water and immediately transfer it to microbiology department.
8.7.4 Add this 2 ml of activated peptone water to a sterile Petri dish and add approximately 20 ml of autoclaved media (Molten). Shake it and allow to solidify.
8.7.5 Incubate the plate at 20° C to 25° C for 72 hour. Then incubate the same plate at 30°C to 35°C for 48 hour for Bacterial Count.
8.7.6 Count the CFU of the plate and Record the observation in report.
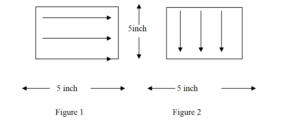
8.7.7 Selection of Surface Area, using one side of moistened swab wipe the test surface of 5” x 5” with 10 firm horizontal strokes as illustrated in figure 1. Turn the swab stick over to its other side, wipe the test surface of 5” x 5” with 10 firm vertical strokes as illustrated in figure 2 . Wherever 5” x 5” area is not available for swab sampling, carry out sampling from hardest to clean area.
8.7.8 Wherever 5” X 5” is not available , carryout sample from hardest to clean area of the entire area .
8.7.9 At the end of the each stroke lift the swab carefully and keep the swab stick in the container.
8.8 Rinse sample and analysis technique
8.9.1 For Chemical:- Use Purified water and collect it into a container. Rinse the equipment & accessories from top to cover all areas of equipment and collect the 100 ml puriedfied water from drain point of equipment. Sample quantity 100ml shall be use for rinse.
8.10 Visual Inspection technique.
8.10.1 Inspect the cleaned equipment by using necessary visual aids (Torch, Lights, etc) while performing the inspection rather than peeping through the glass/ into the machine.
8.10.2 During inspection focus on hard –to- clean areas of the equipments.
8.10.3 Ensure complete dryness of the surface so that all cleaning solutions have been removed and there is no potential for residue formation after wards.
8.11 Un-cleaned equipment hold time
| Stage | Equipment | Time and date of manufacturing process completed (T1) | Time and date of before start of cleaning of equipment (T2) | Observed un cleaned hold time
(T2-T1) |
Hold time of un cleaned equipment. Minimum time (Hrs) |
| After completion of process and before cleaning | Vibro Sifter | 30 Hrs | |||
| Octagonal Blender | 30 Hrs | ||||
| Automatic Capsule Filling Machine | 30 Hrs | ||||
| Blister Packing Machine | 30 Hrs |
8.12 Cleaned equipment hold time
| Stage | Equipment | Time and date of cleaning process completed (T3) | Time and date of hold time of cleaned equipment (T4) | Observed cleaned hold time (T4-T3) | Hold time of cleaned equipment. Minimum |
| After Cleaning Process | Vibro Sifter | 72 Hrs | |||
| Octagonal Blender | 72 Hrs | ||||
| Automatic Capsule Filling Machine | 72 Hrs | ||||
| Blister Packing Machine | 72 Hrs |
9.0 FAILURE INVESTIGATION AND CORRECTIVE ACTION:
If result is comes out of limit then investigate the route cause . On the basis of investigation necessary corrective action shall be implemented. Again repeat the study on worst case for three consecutive run.
10.0 DOCUMENTATION:
10.1 After verifying visual cleanliness, record the observation in report.
10.2 After analyzing the Swab/Rinse samples, record the observation in report.
11.0 REVALIDATION :
11.1 Any major change in equipment
11.2 Any change in cleaning procedure.
113 Change in analytical procedure
11.4 Change in worst case.
11.5 Change in regulatory requirement
11.6 Change in site location.
12.0 CONCLUSION:
Provide the conclusion and recommendation on the basis of execution results of the cleaning validation protocol.
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipment
concurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Protocol for re validation of dry heat sterilizer