sop for Disposal of Scrap
1.0 Objective
To provide necessary guidelines for scrap collection and disposal from the factory premises to avoid
accumulation of waste material in Manufacturing, Warehouse, Packing, Quality control dept.
2.0 Scope
This standard operating procedure is applicable at abc company. It includes disposal of scrap collected in
scrap yard and cut pieces of rejected online packaging material (foils etc.), cut pieces of emptied tubes,
crushed sieves and rejected Die Punch Sets, Bottles, Labels, Engineering Goods etc.
3.0 Responsibility
3.1 All Supervisor of HR & Administration
3.2 Manager/ Head Concern Area
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
HR : Human Resource
CC NO : Change Control Number
cGMP : Current Good Manufacturing Practice
HOD : Head of Department
5.0 Procedure
5.1 Personnel carrying out day to day cleaning operations are trained for collection and disposal of scrap.
5.2 Manufacturing
5.2.1 During / after manufacturing operations, deface labels of active pharmaceutical
ingredient drums/ poly woven bags/ jute bags as well as released slips. Also deface
labels on poly bags containing dispensed raw material.
5.2.2 Transfer these drums & bags to the scrap area.
5.2.3 Collect all other sundry scrap and transfer the same to the scrap area.
5.3 R.M Store
5.3.1 After dispensing deface drum labels of API. Also QC & excipients released label once the drum is empty.
5.3.2 Transfer these drums & bags to the scrap area.
5.3.3 Collect all other sundry scrap and transfer the same to the scrap area.
5.4 Packing
5.4.1 Packing Hall
5.4.2 Packing Area generated scrap like used poly bag, rejected Cartons, rejected Shipper,
Used Stereo, Rejected Caps and Rejected Labels.
5.4.3 Printed packaging material shall be cut into pieces and shredded before disposal.
5.4.4 Collect all rejected carton, label & Shipper other sundry scrap and transfer the same to the scrap area.
5.5 General Administration
5.5.1 Collect all other sundry scrap and transfer the same to the scrap area.
5.6 Quality Control
5.6.1 Collect empty reagent bottles deface their labels and transfer the same to the scrap area.
5.6.2 Product bottles used as samples for analysis, empty out the product,
5.6.3 Collect all other sundry scrap and transfer the same to the scrap area.
5.7 Engineering
5.7.1 Collect used cotton waste, oil, and grease and transfer the same to scrap area in closed poly bags.
5.7.2 Collect all other sundry scrap and transfer the same to the collection site.
5.8 Arrangement of scrap yard: All scrap is classified in four major categories:
1. Recyclable
2. Non-recyclable
3. Hazardous
4. Non-hazardous
5.9 Provide distinctly areas for storage of above mentioned four categories of scrap.
5.10 Put label on each area provided.
5.11 The scrap dealer must have agreement for disposal of scrap duly authorized
by company representative.
5.12 Disposal of the scrap:
5.12.1 Either when the scrap yard is full or on fortnightly basis sells the scrap
through the authorized scrap dealer.
5.12.2 Ensure that all API & additive/diluents vehicle drums are rinsed with potable
water before they are sold as scrap.
5.12.3 Security, administration must check the scrap being disposed.
5.12.4 Allow the scrap to leave the factory premises only after necessary paper work is completed.
5.13 Collect all rejects/scraps generated during various stages of manufacture.
5.14 Differentiate recoverable rejects & non-recoverable rejects.
5.15 Scraps, which can be disposed/ sold on commercial basis, can be sold. Ensure
defacing of labels on empty bulk containers, corrugated boxes etc.
5.16 Collect all rejects/scraps that are to be destroyed in suitable closed containers or suitable
black polybags and label appropriately and store in a segregated space called ‘scrap yard’.
5.17 Record the quantities rejected and destroyed and reconcile in relevant batch documents.
5.18 Destruct all the rejects/scraps as per recommended methods approved by authorized person
under the supervision of a responsible person.
5.19 Ensure appropriate safety precautions like wearing the gloves, safety goggles etc. while carrying out destruction.
5.20 Weigh the total scrap before final disposal and record it.
5.21 Follow the mode of destruction as per chart before disposal.
| S. No. |
Type of Rejections |
Method of Destruction |
| 01 | Empty Containers of Bulk materials from Stores | Deface the labels before disposal. |
| 02 | Powders from dust collection systems | Slurry / Solution in water, or special solvents if necessary. Incineration of solids. |
| 03 | Granules, Powder, Capsules, Tablets. | Slurry / Solution in water, or special solvents if necessary, to be discharges in waste stream (drain).
Incineration of solids. |
| 04 | Strips / Blisters packs including empty packs. | Tablets / Capsules recovered (If permitted according to SOP) by defoiling. Rejected Tablets / Capsules destroyed
Empty Strips / Blisters shredded. |
| 05 | Printed packaging components
(labels, Cartons, foils, leaflets, etc) |
Incineration and/or shredding. |
| 06 | Used stereo figures | Cutting/ shredding/defacing. |
| 07 | Aluminum foils | Crushed using crusher. |
6.0 Forms and Records
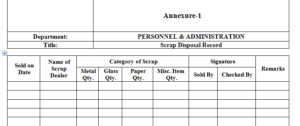
6.1 Scrap Disposal Record – Annexure-1
7.0 Distribution
7.1 Master Copy – Documentation Cell (Quality Assurance)
7.2 Controlled Copy – Quality Assurance, Personnel & Administration, Production
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |
- process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
-
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
sop for Verification of Weighing Balance
-
Sop for Submitting of Leave Application
sop for Accident Management Procedures
sop for Accident Management Procedures
Cleaning and colour coding of Factory Apparel (Clothes)