sop for Technology Transfer
1.0 Objective
To lay down a procedure for technology transfer from R&D and other locations to plant.
2.0 Scope
This Standard Operating Procedure is applicable for technology transfer from R & D and other
locations to plant to be followed at abc Pvt. Ltd.
3.0 Responsibility
3.1 R & D person / other location person shall be responsible for transfer of product manufacturing
technology and related documents to abc location.
3.2 Officer / Executive QA shall be responsible for review of documents received from R&D
and from other location. Officer / Executive QA shall responsible for preparation of other
master documents required for technology transfer.
3.3 Production Manager or his/her designee shall be responsible for monitoring of process as
per formula and necessary arrangement required for technology transfer.
3.4 Head / In charge Q.A. or his/ her designee shall be responsible for compliance of this SOP.
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
QA : Quality Assurance
No. : Number
IPQA : In process Quality Assurance
BPCR : Batch Production and Control Record
R&D : Research & Development
MFR : Master Formula Record
STP : Standard Testing Procedure
5.0 Procedure
5.1 Corporate QA in consultation with R&D shall inform to plant QA / Plant-Head
regarding the transfer of product.
5.2 R&D shall designate competent person for transfer of product and designated person
shall be available at plant at the time of manufacturing.
5.3 R&D person shall hand over the all documents to plant QA related to product as per
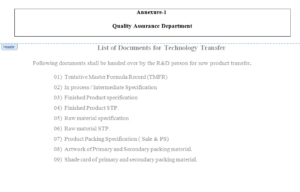
Annexure- 1.
5.4 QA Officer / Executive shall verify the documents as per Annexure-1. If any non
availability of documents found and if any mistakes found, then same shall be informed
to R&D person for correction.
5.5 After receiving the corrected documents, QA Officer / Executive shall distribute the
documents to other concerned departments for reference and necessary arrangements.
5.6 Plant QA Officer / Executive shall prepare the Master BPCR based on TMFR which is
received from R&D.
5.7 QC Officer / Executive shall prepare the RM specifications, PM specification, In process
and Finished Product Specification, STP based on the documents received from R&D.
5.8 Production and R&D person shall ensure the availability of equipments, materials and
Other process requirements for the manufacturing of batch.
5.9 Initial two batches of a product will be considered as technology transfer batches.
5.10 During manufacturing if any changes are done by R&D scientist then same shall be
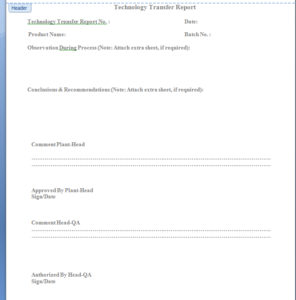
documented using a single deviation form and technology transfer report
(Refer Annexure-2) in consultation with production and QA.
5.11 After successful completion of two batches upto packing stage, technology transfer
report shall be prepared by the R&D as per Annexure-2.
5.12 Analytical methods for raw material, in process and finished product shall be verified by
QC department in presence of R&D scientist.
5.13 Decision for final Authorization of technology transfer report shall be taken plant Head-
QA. If he/ she has the opinion that one or two more batches are required to monitored
then QA-Head shall also recommend the same for taking the batches in the technology
transfer batches.
5.14 Each technology transfer report shall be assigned a unique alphanumeric code which
shall be as TTR/YYY where;
TTR – represents Technology Transfer Report
YYY – denotes serial no. starting from 001 i.e. first technology transfer report number
shall be TTR/001.
5.15 Final signed copy shall be retained by documentation cell- QA and a copy of this shall
be given to R&D.
5.16 On the basis of this technology transfer report R&D will have to revise the TMFR to
incorporate the changes and a copy of final revised TMFR will be circulated to plant QA
for their reference.
5.17 Upon receipt of revised TMFR from R&D, plant QA have to revise their relevant
product BPCR through proper change control procedure as per sop
6.0 Forms and Records
6.1 List of Documents – Annexure- 1
6.2 Technology Transfer Report – Annexure-2
7.0 Distribution:
7.1 Master Copy – Documentation Cell (Quality Assurance)
7.2 Controlled Copy – Quality Assurance , Quality Control , Production & R&D
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |
- process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
-
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
sop for Verification of Weighing Balance
-
Sop for Submitting of Leave Application
sop for Accident Management Procedures
sop for Accident Management Procedures
Cleaning and colour coding of Factory Apparel (Clothes)
Job Responsibilities of Key Personnel
sop for Maintenance of Building
Destruction of Batch Production and Control Records BPCR
Procedure for Sampling of Rinse water Swab
Cleaning Validation of Equipment
Measurement and Recording of Temperature and Relative Humid
Sampling of Product at Intermediate Stages
Approval of Overprinting on Packing Material
sop for Procedure for Reprocessing
Preparation and Control of Master Batch Production and Control record
Issuance and Retrieval of Control documents
Calibration Policy for equipment
procedure for batch numbering system for products