Calibration Policy for equipment
1.0 Objective
To lay down a policy for calibration of equipment, instruments, measuring and monitoring device and their documentation.
2.0 Scope
This Standard Operating Procedure is applicable for applicable for all the
equipment/ instrument / monitoring devices maintained by all departments at formulation plants of abc Pvt. Ltd.
3.0 Responsibility
3.1 Concern department officer / Executive shall be responsible for preparation of calibration planner.
3.2 Head Engineering is responsible for External Calibration & preparation of yearly
External calibration planner for equipment, instrument & monitoring devices.
3.3 Concern Department Head shall be responsible for implementation of this SOP.
3.4 Head QA or his /her designee shall be responsible for compliance of this SOP
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
Calibration : The set of operation which establish, under specified conditions, the relationship
between values indicated by a measuring system, values represented by a material measure
and the corresponding known values of a reference standard.
QC : Quality Control
QA : Quality Assurance
HOD : Head of the Department
5.0 Procedure:
PRECAUTION: Do not use non calibrated equipment / instrument / Devices
5.1 EQUIPMENTS – CALIBRATION & MAINTENANCE
5.1.1 Manufacturing equipment shall be qualified for intended services and shall be assigned
with a unique Tag. No. as its identity. QA Department is responsible for assigning the
Tag No., maintenance of record and the list.
5.1.2 Quality Control / Quality Assurance Department shall perform qualification for
analytical equipment installed in the laboratory. QA department shall assign
Tag No. Independently & shall maintains the record.
5.1.3 Calibration of Engineering / utility equipment / instrument shall be done by
Engineering department. Engineering department is also responsible for calibration of
Measuring Gauges and Timers attached with the equipment or area.
5.1.4 All the equipment has its SOP for operation and calibration procedures and precautions to be followed (if any specific).
5.1.5 Calibration record of equipment is maintained by the user department. The record contains the following:
5.1.5.1 Reference to SOP
5.1.5.2 Calibration Record
5.1.5.3 Acceptance Criteria
5.1.6 Maintenance record of equipment shall be maintained separately.
5.1.7 Calibration & qualification of equipment, apparatus, gauges and recording devices
shall be done at defined frequency. However, when equipment is in operation and for any
other reasons calibration could not be done at due period, in that case calibration
shall be done within the period specified from due date of calibration which shall be as follows:
Set Frequency Permissible Time Period from due date
Daily No Deviation (shall be done on daily basis)
Monthly ±3 Days
Quarterly ±7 Days
Half Yearly ±15 Days
Yearly ±30 Days
5.1.8 Due to any reason, if calibration due date exceeds the above-mentioned permissible
time period, the delay in calibration shall be authorized by Head QA through appropriate deviation report.
5.1.9 When the instrument is not in use due to no activity in the area, daily calibration
of equipment (e.g. Balances, pH Meter etc.) is not necessary. In the calibration record
write No Activity / No Plan / Area Not in Use. However, before start of the activity
in the area, calibration of equipment shall be done and documented.
5.1.10 Any calibration done by external person / party shall be recorded in calibration log
book. The Officer/Executive of respective area shall ensure that equipment is
calibrated before putting into operation.
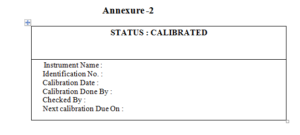
5.1.11 A tag reading, “CALIBRATED” shall be pasted on all equipment after calibration
(Annexure –1). The tag contains information like: /Instrument name, identification No.,
Calibration date, Calibration Done By, Checked By, Next Calibration Due On.
5.1.12 A Calibration planner shall be prepared by Concern dept. (Annexure-2) and shall
be maintained for all the equipments. The Planner shall be periodically reviewed by Section/Department Head.
5.2 External Calibration of Weight AND MEASURING DEVICES
5.2.1 Weights and measuring devices [viz. Reference weights, Thermometers and Measuring]
shall be sent for calibration to external laboratory maintaining National Traceable Reference
for Calibrated Weights and measuring Devices.
5.2.2 After completion of calibration, external party shall issue the ‘Calibration
Certificate’ for each calibrated weight/device. On receipt of such calibration certificate,
user department and engineering dept. shall review the report for accuracy and correctness of the data.
5.2.3 If it is found in compliance, HOD/ In-charge shall put a stamp having details i.e. Reviewed by, Date & Signature.
5.2.4 If the report is not in compliance as per the defined acceptance criteria, further action shall be taken accordingly.
5.2.5 QA department shall review the report randomly during internal audit and put signature on the same.
5.2.6 Except weight boxes / weights, calibration of these devices remains valid for a period of two
years from the date of calibration specified on the calibration certificate. The period of
recalibration is based on handling frequency & storage of these devices. This period is
irrespective of the next calibration due date mentioned on Certificate.
5.2.7 If such calibration could not be arranged in time, the recalibration shall be arranged
within a period of next 3 months. During this extended period, calibration of weight
and measuring devices is considered valid.
5.2.8 Whenever, weights and measuring devices are sent for calibration, the procedure
adopted shall be as follows:
5.2.8.1 Daily calibration of balance shall be carried out only for self-calibration.
If self- calibration features are not available, balances shall be maintained as it is.
During such period, shifting of balances to other place of work is not allowed.
5.2.8.2 If thermometers are sent for calibration, during the period, internally
calibrated (spares) thermometers may be used (if required).
5.2.9 Date in various instruments shall be recorded as per the date system
programmed by instrument/equipment manufacturer.
5.2.10 During breakdown or malfunctioning, the particular instrument to be
identified by a label stating as “OUT OF ORDER” / “UNDER MAINTENANCE”.
5.2.11 When any equipment is out of order:
5.2.11.1 The respective service engineer should be informed.
5.2.11.2 A standby arrangement shall be made if available.
5.2.11.3 If the standby instrument is available, the same may be used after calibration.
5.2.11.4 If standby arrangement is not possible, samples shall sent to other department / external – approved laboratory.
5.2.12 For routine servicing and maintenance, either annual service contract or service
arrangement should be made with instrument manufacturer/ authorized service agent.
5.2.13 A history card of all critical & major equipment shall be maintained by the user
department. A service report of the same should maintain separately in the dept.
5.3 HANDLING OF STANDARD CALIBRATED WEIGHTS
5.3.1 On the receipt of calibrated standard weights, engineering dept. shall ensure that
all relevant calibration certificates are obtained in original.
5.3.2 Whenever, weights and measuring devices are sent for calibration, the procedure adopted shall be as follows.
5.3.3 These calibration certificates shall be retained by user dept. This certificate shall
be traceable and may be crossed checked by QA personnel at any time.
5.3.4 The analytical standard weight box up to 200 gm shall be kept properly in
secured place and shall be maintained in original box.
5.3.5 All standard weights shall be wrapped lying in a double LDPE bags and
should be stored in Plastic / Stainless steel box.
5.3.6 All standard calibrated weights (cast Iron) used in Stores; Tablet & Capsule
department shall be kept on Pallets / Trolley. These weights are covered with
double LDPE bags so as to prevent dust deposition. Display the calibration certificate,
including date of calibration and due date of calibration near pallet / trolley.
5.3.7 Handling of analytical weights (up to 200 gm) shall carefully be done by using plastic tip forceps / gloves.
6.0 Forms and Records
6.1 Calibration planner : Annexure-1
6.2 Label for calibration status : Annexure-2
7.0 Distribution
7.1 Master Copy : Documentation Cell (Quality Assurance)
7.2 Controlled Copy : Quality Control, Production, Warehouse, Engineering & HR.
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |

- process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
-
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
sop for Verification of Weighing Balance
-
Sop for Submitting of Leave Application
sop for Accident Management Procedures
sop for Accident Management Procedures
Cleaning and colour coding of Factory Apparel (Clothes)
Job Responsibilities of Key Personnel
sop for Maintenance of Building
Destruction of Batch Production and Control Records BPCR
Procedure for Sampling of Rinse water Swab
Cleaning Validation of Equipment
Measurement and Recording of Temperature and Relative Humid
Sampling of Product at Intermediate Stages
Approval of Overprinting on Packing Material
sop for Procedure for Reprocessing
Preparation and Control of Master Batch Production and Control record