sop for handling of re-test materials
1.0 Objective
To lay down a procedure for handling of re-test materials.
2.0 Scope
This Standard Operating Procedure is applicable for handling of re-test materials to be followed
at formulation plants of abc company.
3.0 Responsibility
3.1 Officer/Executive warehouse shall be responsible for handling of re-test materials.
3.2 Head Warehouse is responsible for ensuring the compliance of the SOP.
3.3 Head QA or his designee shall be responsible for implementation and compliance with SOP.
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
i.e. : That is
No. : Number
w.r.t : With respect to
WH : Warehouse
HOD : Head of Department
5.0 Procedure
5.1 Handling of retesting of raw material
5.1.1 Warehouse Officer / Executive shall prepare the monthly list in the last week of every month
for all the raw materials, which are due for retesting in the next month and intimate to Quality Control Department
as per format No
5.1.2 Prepare intimation slips for as per format and send to QC Department.
5.1.3 Deface / cross the existing approved label for all the materials for which retesting is required.
5.1.4 Put quarantine label and transfer the material to the designated quarantine area.
5.1.5 QC shall take sample from the material and affix sample and under test label on the
container from which the sample has been taken.
5.1.6 Transfer the sampled material into the designated under test area.
5.1.7 After testing, the QC department shall paste new approved / rejected label with new A.R. No. and retest date.
5.1.8 The material shall be transferred from under test material to approved/rejected material Area.
5.2 Handling of near expiry raw materials
5.2.1 Warehouse shall give details of the material near to expiry to plant head.
5.3 Handling of expired raw materials
5.3.1 Cross the approved labels of the material.
5.3.2 Transfer expiry material in rejected area.
5.3.3 Take the permission of destruction of raw material on destruction note and get it approval
from QA and plant head and destroy the material as per suggested mode in the presence of QA and Security Personnel.
6.0 Forms and Records
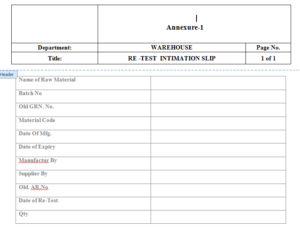
6.1 Re -Test Intimation Slip – Annexure-1
7.0 Distribution
7.1 Master Copy – Documentation Cell (Quality Assurance)
7.2 Controlled Copy – Warehouse Department.
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |
- process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
sop for Dispensing of Raw Material
Receipt of Excess RM/PM from Production
sop for Dispatches of Finished Goods
Cleaning of Dispensing and Sampling Area
procedure for cleaning of dispensing tools
Cleaning and Operation of Reverse Laminar Air Flow
Handling of Open-Damaged Containers in Warehouse
sop for Discard of Scrap From Warehouse
Cleaning and Operation of Reverse Laminar Air Flow
sop for Cleaning of High Racks
sop for Issuance of Excess RM/PM to Production
sop for Cleaning of MS Shutter
procedure for handling of rejected materials