sop for Preparation and Approval of List of Authorized personnel
1.0. OBJECTIVE :
1.1 The objective of this sop is to define the procedure for Preparation of List of Authorized Personnel.
2.0. SCOPE :
2.1 This procedure is applicable to Preparation of List of Authorized Personnel of concerned department
3.0. RESPONSIBILITY :
3.1 Officer/ executive of user department shall responsible for preparation of list.
3.2 Head of department or designee shall be responsible for checking SOP.
4.0. ACCOUNTABILITY :
Head QA.
5.0. PROCEDURE :
5.1. To prevent entry of Unauthorized Personnel in Critical Areas , All Department shall prepare
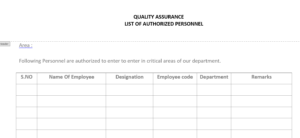
list of Authorized personnel as per format “List of Authorized Personnel” as shown in Annexure-I..
5.2. “Authorized personnel” means that the person signing the document, should have sufficient knowledge,
training & expertise To adequately review & approve date, information, conclusion & recommendation contained therein.
5.3. User department shall prepare the list of authorized personnel and sent to Department Head for checking.
5.4. User department Head after checking sent the list of Authorized Personnel to QA department for approval of the list.
5.5. Head QA shall approve the list of Authorized personnel.
5.6. If there is any change in staff either by resign or by transfer to other location or department , concerned
department shall update the list of Authorized Personnel .
5.7. User Department Head, Head QA are authorized to Access in all Areas.
5.8. Visitors & Vendors are allowed to enter in Permitted Areas only, if they are accompanied with any of
Authorized Person of Concerned Area.
5.9. Microbiologists are authorized to enter in all Critical Areas for Environmental Monitoring .
5.10. Engineering Personnel are allowed to enter in Critical Areas for Maintenance work, if they are
accompanied with any of Authorized Person of Concerned Area.
5.11. User department shall update the Authorized Personnel List after every three months.
6.0 ABBREVIATION :
| Sr. No. | Abbreviation used | Full form of abbreviation used |
| 1. | SOP | Standard Operating Procedure |
| 2. | QA | Quality Assurance department |
7.0 ATTACHMENTS (ANNEXES) : –.
Annex-I : List of Authorized Personnel
8.0 REFERENCE :
| Sr. No. | Reference Title |
| 1.0 | In house |
Annex-I : List of Authorized Personnel
sop for Preparation and Approval of List of Authorized personnel
sop for handling of external audits
sop for Checking of Proof and Overprinting
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipment
concurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection