sop for Submitting of Leave Application
1.0 Objective
To lay down the procedure for submitting of leave application.
2.0 Scope
This SOP is applicable for submitting of leave application at abc company.
3.0 Responsibility
3.1 All Supervisor of HR & Administration
3.2 Manager/ Head Concern Area
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
HRD : Human Resource Department
CC NO : Change Control Number
cGMP : Current Good Manufacturing Practice
HOD : Head of Department
5.0 Procedure
5.1 Procedure Leave Application
5.1.1 Employee who required leave fill the leave application form provided by the HR department.
5.1.2 Department head sign the leave application, approval of leave Depends on the department
head as well as the strength of employee working in the department.
5.1.3 After approval of leave by the department head HR head will sign the leave application.
5.1.4 If the leaves are not remaining in the leave account of the employee, approval of leave
depends on the HR head.
5.1.5 After approval of HR Head, GM plant will sign the leave application.
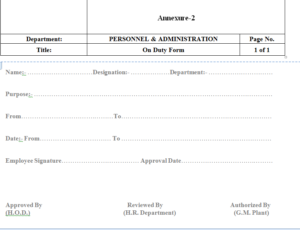
5.2 Procedure On Duty Form
5.2.1 Employee who required OD or going to outside the company for company work fill the
OD form provided by the HR department.
5.2.2 Department head sign the OD form.
5.2.3 After approval of OD by the department head HR head will sign the OD Form.
5.2.4 After approval of HR Head , GM Plant will sign the OD form,
6.0 Forms and Records
6.1 Leave Application Form – Annexure-1
6.2 On Duty Form – Annexure-2
7.0 Distribution
7.1 Master Copy – Documentation Cell (Quality Assurance)
7.2 Controlled Copy – Quality Assurance, Personnel & Administration.
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |

- process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
-
process validation protocol for methylcobalamin niacinamide and pyridoxine injection
validation protocol of sterility test
sop for Analytical Method Transfer
Protocol for hold time study of sterile garments
sop for swab sampling for validation of clean surfaces
cleaning validation maco and noel calculation formula
sop for performance qualification for analyst
Sop for Validation report for disinfectant efficacy
Sop for Method validation report for bacterial endotoxin test
Sop for method validation microbiology sterility testing
sop for validation report for preservative efficacy test
sop for Protocol cum report for efficacy qualification of uv light
sop for validation protocol for uv light efficacy of dpb & laf
sop for cleaning validation protocol tablet manufacturing equipment
sop for cleaning validation protocol ointment manufacturing equipmentconcurrent process validation for rabeprazole ec and domperidone sr capsules
sop for Validation for cleaning procedure liquid injection
sop for Validation for cleaning procedure dry powder injection
Preparation Approval Control and Distribution of Master Formula Records
calibration policy for equipment and instruments
evaluation Sampling of Raw Materials questionnaire
training evaluation questionnaire
sop for approval of Contract Parties
sop for Operation and Cleaning of Purified Water Generation System
sop for Storage of Standard Weights
sop for Verification of Weighing Balance
-