sop for non conformance of RM PM and finished product
1.0. OBJECTIVE:
The objective of this SOP is:
1.1 To describe a procedure for Handling of Non Conformance (NCR) of Raw Material (RM) / Packaging Material (PM) and Finished Product (FP).
1.1.1 Raw material / finished product which conforms to Regulatory specifications but does not conform to in house specifications.
1.1.2 Non- critical defects in packaging material, which does not affect finished product quality.
2.0. RESPONSIBILITY:
2.1 Head of the Concerned Department shall be:
2.1.1. Responsible for Non-Conformance report.
2.2 Quality Assurance shall be:
2.2.1 Responsible for investigating and approving the Non-Conformance Report.
3.0. ACCOUNTABILITY:
Head – Quality Assurance
4.0. PROCEDURE:
4.1 On receipt of the rejection test reports of the Raw material / Packaging materials / Finished products from
the Quality Assurance Dept., the Dept. Head of the affected Department shall decide whether a request can be made for review of rejection decision.
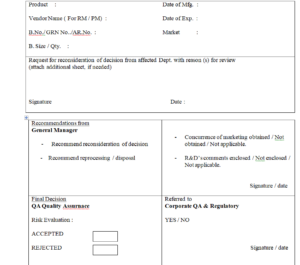
4.2 The request for review shall be made in triplicate in Non- conformance Report (NCR) as per Annexure – 1
4.3 The Head of the affected Dept. or his representative shall specify the reason (s) for review in the NCR. The affected Dept. shall
send the NCR to General Manager for his recommendation to.
4.4 Quality Assurance – Head shall decide to Accept or Reject the NCR based on the risk evaluation.
4.5 If necessary, Quality Assurance – Head before taking the final decision may consult Research & Development Head / President – Technical Operations.
4.6 On absence of Quality Assurance Head, President – Technical Operations shall decide to Accept or Reject the NCR.
4.7 The NCR shall be distributed for final disposal as under, the original NCR shall be filed in the Documentation room
along with the QC report of the respective Batch / AR no.
4.8 One photocopy shall be forwarded to the Head of the originating Dept. for reference.
4.9 One photocopy shall be retained by QA for Annual Product Review.
5.0. REASON FOR REVISION:
This SOP is revised due to revision due date.
6.0. TRAINING:
Trainer — Head – Quality Assurance
Trainee — All departmental heads / Quality Assurance Personnel
Period — One day
7.0. DISTRIBUTION:
Certified Copy No. 1 : Head of Department – Quality Control
Certified Copy No. 4 : Head of Department – Warehouse
Certified Copy No. 5 : Head – Plant Operations
Original Copy : Head – QUALITY ASSURANCE
8.0. ANNEXURE:
Nil.
9.0. REFERENCE:
In-House
ANNEXURE – 1
FORMAT FOR NON-CONFORMANCE REPORT
NON-CONFORMANCE REPORT
(For Non-Regulatory Reasons Only)
sop for Calibration and Maintenance of Laboratory Instruments and Equipment
Disposal of Residual Sample or Left Over Material
sop for for Laboratory Incident
standard operating procedure temperature monitoring
sop for operation of infrared moisture balance
sop for preparation of mobile phase
sop for Preparation and Issuance of Analysis protocol standard
sop of placebo and impurity stock solutions
sop for disposal of residual sample
sop for handling of pharmacopoeial changes
sop for procedure for operation of ultrasonic cleaner
difference between UPLC and HPLC
sop for for Emergency Eyewash and Shower
sop for operation and calibration of total organic carbon analyzers
sop for operation of cobb tester
sop for Operation and calibration of atomic absorption spectrophotometer
sop for Operation and calibration of gas liquid chromatograph
sop for operation of humidity oven
sop for operation and calibration of serological water bath
sop for monitoring of drain trap
sop for destruction of analytical samples after testing and control samples
sop for destruction of used chemicals
Sop for Operation of suction pump
sop for Operation and calibration uv cabinet
sop for Operation and calibration of bulk density apparatus
sop for operation and calibration of shore hardness tester
sop for operation of rub proofness tester
sop for monitoring of purified water
sop for Retesting of packaging materials
sop for Retesting and resampling of raw materials
sop for Control of issuance of record of analysis green sheets
sop for Control of computer passwords
sop for sampling of packaging materials PM
sop for sampling of sterile raw material
sop for sampling of intermediates and finished products
sop for operation and calibration of friability test apparatus
sop for approval and rejection of packaging materials
sop for non conformance of RM PM and finished product
sop for collection storage and disposal of control samples
sop for trend analysis of finished products
sop for Chromatographic practices and system suitability
SOP For Good Laboratory Practices
sop for cleaning and operation of sieve shaker
general specification of packing material cartons
sop for Password for Analytical Instrument and LIMS software
sop for Rounding off numerical analytical results
sop for sampling of bulk and finished product
sop for cleaning of spillage material
sop for Handling of Reference Standard
sop for hplc column maintenance and washing
procedure for sampling and handling of bulk sample
STP for borewell generation point (raw water storage tank)
sop for preparation and standardization of 0.1M Zinc Sulphate
Operation &calibration of analytical balance (dhona)
Operation and Calibration Procedure for Disintegration Test apparatus
sop for preparation and standardization of 1 M Hydrochloric Acid
Preparation and standardization of 0.1 M ceric ammonium sulphate solution
sop for preparation and standardization of 0.05 m iodine solution
validation of volumetric solution 0.1m ammonium thiocyanate
handling of reference standard and preparation of working standard
sop for water sampling and analysis
sop for operation for validation of excel worksheets
sop for stability of volumetric solutions
sop for preparation of raw material in process finish product packing material data sheets
sop for handling of hazardous chemicals
sop for handling of glassware and allocation of identification number
sop for operation cleaning and calibration of bursting strength tester
sop for rounding off the analytical test results
procedure for Analyst Qualification
sop for operation and calibration of dissolution Apparatus
procedure for maintenance of desiccators
sop for for hplc column receipt checking id no and regeneration
safety data sheet for laboratory chemicals
procedure for handling of poisonous chemicals
sop for cleaning of sampling devices
sop for calibration procedure of instruments
sop for specification and standard testing raw material packing material and finished product
procedure for operation and calibration of potentiometric titrator
procedure for operating and calibration of digital hardness tester
procedure for disposal of expired chemicals, reagents and solvents
sop for behavior in quality control department
sop for preparation and standardization 0.1M sodium thiosulphate
sop for preparation and standardization 0.1M Disodium Edetate
preparation and standardization 0.1M Sodium Hydroxide Solution
Preparation and standardization of 0.1M Perchloric acid solution
sop for preparation 0.05M edetate disodium
sop for preparation 0.1M silver nitrate
sop for Operation and Calibration of High Performance Liquid Chromatography
sop for UV & Visible Spectrophotometer
procedure for Cleaning of laboratory glassware
Cleaning of Instrument, Instrument bench and surrounding area of Quality
Safety Precaution in Quality Control Department
Operation & Calibration of Analytical Balance
Calibration of Glassware in Quality Control Department
handling of samples received in Quality Control
Cleaning and Operation of Refrigerator
Operation, Cleaning and Calibration of water bath
Operation & Calibration of Refractometer
Operation and Cleaning of Centrifuge Apparatus
cleaning, operation & calibration of Vernier caliper
Calibration of Fourier Transform Infrared Spectrophotometer (FTIR)
Cleaning and operation of Moisture Analyzer
Cleaning & Operation of Vacuum pump in Quality Control Department
Operation and Calibration of Polarimeter
Cleaning and operation of Magnetic Stirrer
Cleaning Operation and Calibration of Melting Point
Operation Cleaning and Calibration of Muffle Furnance in Quality Control Department
procedure of operation and Cleaning of Sonicator
Operation Cleaning & Calibration of pH meter in Quality Control Department
Entry and Exit in Quality Control Department