sop for for Laboratory Incident
1.0 OBJECTIVE:
To lay down a procedure for Laboratory Incident.
2.0 SCOPE:
This SOP is applicable of procedure for Laboratory Incident in quality control department
3.0 RESPONSIBILITY:
Executive/Officer QC/designee.
4.0 ACCOUNTABILITY:
Head- Quality Control
5.0 PROCEDURE
5.1 Standard Practices:
5.1.1 During analysis before reporting if Incident found as per points listed below shall be considered as Laboratory Incident and Incident shall be logged in Annexure-I and filled in Annexure – II of the SOP.
5.1.2 Following points shall be considered in Laboratory Incident.
5.1.3 General Incident :
5.1.3.1 Borderline results due to analyst error or Instrument error.
5.1.3.2 Tested parameter other than given in specification.
5.1.3.3 Mistake in calculation and or reporting.
5.1.3.4 Any contamination during sample / standard preparation / storage of sample.
5.1.3.5 STP deviation with respect to weighing, dilutions, standards etc.
5.1.3.6 Improper sample handling during analysis.
5.1.3.7 Glassware breakage with sample or standard.
5.1.3.8 Discontinue analysis for electrical problems, urgent re-planning of work.
5.1.3.9 Entry missing in instrument/other respective log book.
5.1.4 Chromatography
5.1.4.1 Injection Carryover
5.1.4.2 Shift in Retention Time / Relative Retention Time (Not more than 10 %)
5.1.4.3 Improper Peak Shape and Peak Splitting
5.1.4.4 Baseline Drift
5.1.4.5 Bracketing standard does not meet acceptance criteria.
Note: In case if complete batch table run and chromatogram obtained then above points shall be considered as laboratory Incident.
5.1.5 Spectroscopy:
5.1.5.1 Extra peaks in the spectrum
5.1.5.2 Low Correlation with standard spectrum
5.1.6 Microbiology:
5.1.6.1 Wrong media preparation
5.1.6.2 Other unplanned unwanted event.
5.1.7 Instrument malfunctioning
5.1.7.1 Column Issue
5.1.7.2 Software malfunctioning
5.1.7.3 Power Failure
5.1.7.4 Hardware Error
5.1.7.5 Leak Detection
5.1.7.6 Communication error
5.1.7.7 Frit / Cartridge choke
5.1.8 Analyst Oversight
5.1.8.1 Wrong material
5.1.8.2 Wrong glassware
5.1.8.3 Glassware cleaning
5.1.8.4 Instrument out of calibration
5.1.8.5 Reviewer oversight
5.1.8.6 Entry error
5.1.8.7 Sample / Standard preparation error
5.1.8.8 Sample spillage during the analysis
5.1.8.9 Wrong sequence on instrument error
5.1.9 Based upon sound scientific principles, analyst shall discontinue testing and immediately notify
supervisor or designee if an incident, problem or error is suspected or recognized.
5.1.10 Allocation of Analytical reference number for Laboratory Incident:
5.1.10.1 Laboratory Error shall be given the specific number as QC/INC/XX/YYY, where ‘XX’ shall be for year and ‘YYY’ shall be Serial number as ‘001.
Where is QC for Quality Control, INC for Incident, XX for current year and YYY shall be Serial number as ‘001. (e.g. QC/INC/21/001)
5.1.11 In case of Laboratory Incident conforms before reporting OOS shall be not applicable.
5.1.12 In Investigation no any Probable /Root cause identified OOS shall be applicable
5.1.13 All Incidents shall be logged by QC dept. as per respective Annexure.
5.1.14 After Investigation with corrective action & Preventive action, analysis repeat the sample.
5.1.15 Conclusion of QA Head shall be completed after re-analysis the sample and observed the result.
5.1.16 If any Chromatogram system suitability parameters does not complies or any discrepancy in running system been detected online,
the same should be made invalid and maintain the record as per Annexure-III
5.1.17 PROCEDURE FOR DATA INVALIDATION: Data shall be invalidated in the following cases when any of the below error may identified online:-
5.1.17.1 Wrong Batch Table
5.1.17.2 Peak Splitting
5.1.17.3 System suit failure at any injection
5.1.17.4 Improper peak shape
6.0 ABBREVIATION:
Ltd. :Limited
SOP : Standard Operating Procedure
No. :Number
QC :Quality Control
QA :Quality Assurance
STP :Standard Operating procedure
RSD :Relative Standard Deviation
HPLC :High Performance Liquid Chromatography
TOC :Total Organic Carbon
7.0. ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Laboratory Incident Log Book |
| Annexure-II | Laboratory Incident Form |
| Annexure-III | Inward for Invalid Documents |
8.0 DISTRIBUTION:
Controlled Copy : Quality Control Department
Master Copy : Quality Assurance Department
9.0 REFERENCES:
In House
10.0 REVISION HISTORY:
| Revision No. | Change Control No. | Details of Changes | Reason of Changes | Effective Date | Done By |
| 00 | Not Applicable | Not Applicable | New SOP |
ANNEXURE-I
Laboratory Incident Log Book
| S.
No |
Date | Incident No. | Product/ Material Name | Batch No. | Name of Analyst | Description of Incident | Initiated by | Checked by | Rem ark |
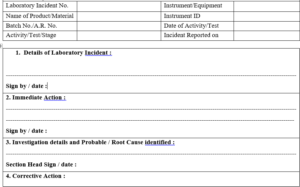
ANNEXURE-II
Laboratory Incident Form

ANNEXURE – III
INWARD FOR INVALID DOCUMENTS
| S. No. | Date | Product/ Material Name | Batch No/AR No. | Instrument Name/ID | Reason for Invalid | Corrective Action | Analyst Name | Checked By/Date | Remarks |
sop for Calibration and Maintenance of Laboratory Instruments and Equipment
Disposal of Residual Sample or Left Over Material
sop for for Laboratory Incident
standard operating procedure temperature monitoring
sop for operation of infrared moisture balance
sop for preparation of mobile phase
sop for Preparation and Issuance of Analysis protocol standard
sop of placebo and impurity stock solutions
sop for disposal of residual sample
sop for handling of pharmacopoeial changes
sop for procedure for operation of ultrasonic cleaner
difference between UPLC and HPLC
sop for for Emergency Eyewash and Shower
sop for operation and calibration of total organic carbon analyzers
sop for operation of cobb tester
sop for Operation and calibration of atomic absorption spectrophotometer
sop for Operation and calibration of gas liquid chromatograph
sop for operation of humidity oven
sop for operation and calibration of serological water bath
sop for monitoring of drain trap
sop for destruction of analytical samples after testing and control samples
sop for destruction of used chemicals
Sop for Operation of suction pump
sop for Operation and calibration uv cabinet
sop for Operation and calibration of bulk density apparatus
sop for operation and calibration of shore hardness tester
sop for operation of rub proofness tester
sop for monitoring of purified water
sop for Retesting of packaging materials
sop for Retesting and resampling of raw materials
sop for Control of issuance of record of analysis green sheets
sop for Control of computer passwords
sop for sampling of packaging materials PM
sop for sampling of sterile raw material
sop for sampling of intermediates and finished products
sop for operation and calibration of friability test apparatus
sop for approval and rejection of packaging materials
sop for non conformance of RM PM and finished product
sop for collection storage and disposal of control samples
sop for trend analysis of finished products
sop for Chromatographic practices and system suitability
SOP For Good Laboratory Practices
sop for cleaning and operation of sieve shaker
general specification of packing material cartons
sop for Password for Analytical Instrument and LIMS software
sop for Rounding off numerical analytical results
sop for sampling of bulk and finished product
sop for cleaning of spillage material
sop for Handling of Reference Standard
sop for hplc column maintenance and washing
procedure for sampling and handling of bulk sample
STP for borewell generation point (raw water storage tank)
sop for preparation and standardization of 0.1M Zinc Sulphate
Operation &calibration of analytical balance (dhona)
Operation and Calibration Procedure for Disintegration Test apparatus
sop for preparation and standardization of 1 M Hydrochloric Acid
Preparation and standardization of 0.1 M ceric ammonium sulphate solution
sop for preparation and standardization of 0.05 m iodine solution
validation of volumetric solution 0.1m ammonium thiocyanate
handling of reference standard and preparation of working standard
sop for water sampling and analysis
sop for operation for validation of excel worksheets
sop for stability of volumetric solutions
sop for preparation of raw material in process finish product packing material data sheets
sop for handling of hazardous chemicals
sop for handling of glassware and allocation of identification number
sop for operation cleaning and calibration of bursting strength tester
sop for rounding off the analytical test results
procedure for Analyst Qualification
sop for operation and calibration of dissolution Apparatus
procedure for maintenance of desiccators
sop for for hplc column receipt checking id no and regeneration
safety data sheet for laboratory chemicals
procedure for handling of poisonous chemicals
sop for cleaning of sampling devices
sop for calibration procedure of instruments
sop for specification and standard testing raw material packing material and finished product
procedure for operation and calibration of potentiometric titrator
procedure for operating and calibration of digital hardness tester
procedure for disposal of expired chemicals, reagents and solvents
sop for behavior in quality control department
sop for preparation and standardization 0.1M sodium thiosulphate
sop for preparation and standardization 0.1M Disodium Edetate
preparation and standardization 0.1M Sodium Hydroxide Solution
Preparation and standardization of 0.1M Perchloric acid solution
sop for preparation 0.05M edetate disodium
sop for preparation 0.1M silver nitrate
sop for Operation and Calibration of High Performance Liquid Chromatography
sop for UV & Visible Spectrophotometer
procedure for Cleaning of laboratory glassware
Cleaning of Instrument, Instrument bench and surrounding area of Quality
Safety Precaution in Quality Control Department
Operation & Calibration of Analytical Balance
Calibration of Glassware in Quality Control Department
handling of samples received in Quality Control
Cleaning and Operation of Refrigerator
Operation, Cleaning and Calibration of water bath
Operation & Calibration of Refractometer
Operation and Cleaning of Centrifuge Apparatus
cleaning, operation & calibration of Vernier caliper
Calibration of Fourier Transform Infrared Spectrophotometer (FTIR)
Cleaning and operation of Moisture Analyzer
Cleaning & Operation of Vacuum pump in Quality Control Department
Operation cleaning and calibration of Karl Fischer Apparatus
Operation and Calibration of Polarimeter
Cleaning and operation of Magnetic Stirrer
Cleaning Operation and Calibration of Melting Point
Operation Cleaning and Calibration of Muffle Furnance in Quality Control Department
procedure of operation and Cleaning of Sonicator
Operation Cleaning & Calibration of pH meter in Quality Control Department
Entry and Exit in Quality Control Department