Analysis of Glimepiride and Metformin Hydrochloride (SR) Tablets
Description : Take 10 tablets, observed visually & record.
Identification As per Assay procedure: Positive test for Glimepiride & Metformin Hydrochloride.
Average Weight : Weigh accurately 20 tablets, note down the weight and divide the gross weight by 20 to obtain
average weight of tablet.
Uniformity of weight : Weigh 20 tablets selected at random and determine the average weight.
Not more than two of the individual weights deviate from the average weight by more than the percentage deviation shown in Table I and none deviates by more than twice that percentage.
Table-I
| Average weight of tablets | Percentage deviation |
| 80 mg or less | 10 |
| More than 80 mg and less than 250 mg | 7.5 |
| 250 mg or more | 5 |
Uniformity of content For Glimepiride :
Chromatographic Condition:
Column : C-18 (250 mm X 4.6 mm) 5 micron
Flow rate : 1 ml per minute
Wavelength : 228 nm nm
Injection Volume : 20 ml
Column Temp. : Ambient
Mobile phase: Prepared a mixture of 35 volumes of a buffer solution ( 0.5 gm monobasic sodium
phosphate in 500 ml of water, adjust to pH 2.1 to 2.7 with orthophosphoric acid) and 65 volumes
of Acetonitrile and filter through 0.45 micron filter & degassed.
Standard Preparation: Taken 30 mg of Glimepiride working standard in a 100 ml volumetric flask add 50 ml solvent mixture dissolve and volume up to 100 ml with solvent mixture. Further dilute 5 ml of this solution to 50 ml with solvent mixture and filter through 0.2 micron filter.
Sample preparation:
Taken randomly 10 tablets for analysis.
Place 1 tablet in a 100 ml volumetric flask add 20 ml Solvent mixture disperse and dissolve and volume up to 50 ml with Solvent mixture. Filter the above solution in a test tube. Take 5 ml of this solution in a 50 ml and further dilute 50 ml with Solvent mixture mix and filter through 0.2 micron filter. . Same procedures repeat 9 other tablets.
Procedure: Separately inject 20 ml. of five injection of standard and two injections of sample preparations into liquid chromatography and record the chromatograms. Measure the responses for major peak. Test is not valid if theoretical plates are less than 1000, tailing factor is more than 2.0 and RSD is more than 2.0%.
Calculate the content of Glimepiride from the principal peak areas of Standard and sample preparations and percentage assay of working standard used.
Calculation :
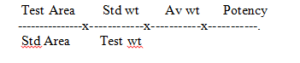
Drug release test for Metformin Hydrochloride:
Apparatus : Paddle.
Medium : 900 ml. of water.
Time : 60 minutes, 180 Minutes & 420 minutes
Speed : 100 RPM
Temperature : 37oC ± 0.5oC
Standard dilution: Taken 110 mg of Metformin Hydrochloride working standard in 100 ml volumetric flask add 70 ml of water dissolve and volume up to 100 ml with water. Further dilute 2 ml of this solution to 200 ml with water.
Sample preparation: Set dissolution parameters using 900 ml dissolution medium. Place one tablet in to each bowl and lower down the hood taking care to exclude air bubbles from the surface of the tablet and immediately start the apparatus. At the end of 60 minutes, 180 minutes & 420 minutes withdraw 20 ml sample & filter. Transfer the 2 ml filtrate solution to 200 ml with water.
Procedure: Measure the Standard solution and sample solution 232 nm using water as a blank.
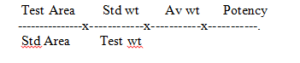
Assay:
Each uncoated bilayered tablet contains:
For Glimepiride (by HPLC):
Chromatographic Condition:
Column : C-18 (250 mm X 4.6 mm) 5 micron
Flow rate : 1 ml per minute
Wavelength : 228 nm nm
Injection Volume : 20 ml
Column Temp. : Ambient
Mobile phase: Prepared a mixture of 35 volumes of a buffer solution ( 0.5 gm monobasic sodium phosphate in 500 ml of water, adjust to pH 2.1 to 2.7 with orthophosphoric acid) and 65 volumes of Acetonitrile and filter through 0.45 micron filter & degassed.
Solvent mixture: A mixture of 90 volumes of Acetonitrile and 10 volumes of water.
Standard dilution: Taking 30 mg of Glimepiride working standard in a 100 ml volumetric flask add 20 ml solvent mixture dissolve and volume up to 100 ml with solvent mixture. Further dilute 5 ml to 50 ml with solvent mixture and filter through 0.2 micron filter.
Sample dilution: Taking 30 mg of Glimepiride tablet powder in a 100 ml volumetric flask add 20 ml solvent mixture dissolve and volume up to 100 ml with solvent mixture and filter then. Further dilute 5 ml filtrate solution to 50 ml with solvent mixture and filter through 0.2 micron filter.
Procedure: Separately inject 20 ml. of five injection of standard and two injections of sample preparations into liquid chromatography and record the chromatograms. Measure the responses for major peak. Test is not valid if theoretical plates are less than 1000, tailing factor is more than 2.0 and RSD is more than 2.0%.
Calculate the content of Glimepiride from the principal peak areas of Standard and sample preparations and percentage assay of working standard used.
Calculation :
For Glimipride:
Spl Area. Std. wt. 5 100 50 P
————X———-X——-X———–X———X——– X avg. wt.
Std. Area. 100 50 Spl wt 5 100
Where;
P= Potency of working standard
For Metformin Hydrochloride (by UV):
Standard dilution: Taken accurately 50 mg of Metformin Hydrochloride working standard in 100 ml volumetric flask add 70 ml water dissolve and volume up to 100 ml with water. Further dilute 1 ml of this solution to 50 ml with water.
Sample dilution: Taken accurately 50 mg of Metformin Hydrochloride tablets powder in 100 ml volumetric flask add 70 ml water dissolve and volume up to 100 ml with water mix and filter. Further dilute 1 ml of this filtrate solution to 50 ml with water.
Procedure: Measure the Standard solution and sample solution 232 nm using water as a blank.
Spl Abs. Std. wt. 1 100 50 P
————X———-X——-X———–X———X——– X avg. wt.
Std. Abs. 100 50 Spl wt 1 100
Where;
P= Potency of working standard
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
analysis of Procyclidine HCl 5 mg Tablet
analysis of silodosin 8 mg capsule
Method of Analysis Ceftriaxone and Sulbactam For Injection
Analysis of Glimepiride and Metformin Hydrochloride (SR) Tablets