standard testing procedure dextromethorphan hydrobromide
Storage Requirements:
Store protected from light and moisture.
Sampling:
Sample equal quantity from each container / bag. Collect a minimum of 5g from each container/bag sample into individual, self –sealing clear polythene bag kept in another clear self sealing polythene bag bearing ‘Sample for Analysis,lable.After completion of sampling return rest sample on same container. Collect control sample in Pet Bottle/Glass Bottle.
Quantity of Composite Sample:
15 g
Quantity of individual Identification
0.0100 g from each container /bag.
Quantity of Control Sample:
2 X 15 g
Description: An almost white crystalline powder.
Solubility: Freely soluble in ethanol (95 per cent) and in chloroform; sparingly soluble in water; practically
Insoluble in ether.
Identification
:
Test A may be omitted if tests B, C and D are carried out. Tests B and C may be omitted if tests A and D are carried out.
A. By IR: Determine by infrared absorption spectrophotometer. Compare the spectrum with that obtained with
Dextromethorphan HBr RS.
B. An absorption maximum only at about 278nm.
C. In the test for related substances, the principal peak in the chromatogram obtained with test solution corresponds to that in the chromatogram obtained with reference solution.
D. Gives the reaction of bromides.
Appearance of solution: clear and colourless.
Acidity or alkalinity: The solution is yellow and not more than 0.4ml of 0.01M hydrochloric acid is required to change the colour to red.
Specific optical rotation: +28.0° to +30.0
N,N-Dimethylaniline: The resulting solution is not more intensely coloured than that obtained by treating at the same time and in the same manner a solution containing 5µg of N, N-dimethylaniline in 20ml of water.
Related Substances: Determine by liquid chromatography
I) Single Maximum Impurity: NMT – 0.5%.
II) Any other Impurity: NMT – 0.25%.
III) Total Impurity: NMT – 1.0%.
Sulphated Ash: NMT 0.1%w/w.
Water: 4.0% to 5.5%w/w.
Assay: 99.0% to 101.0%w/w on anhydrous basis.
Description: An almost white crystalline powder.
Reporting: Report as Complies/Does not comply.
Solubility: Freely soluble in ethanol (95 per cent) and in chloroform, sparingly soluble in water, practically insoluble in ether.
Reporting: Report as Complies/Does not comply.
Identification:
Test A may be omitted if tests B, C and D are carried out. Tests B and C may be omitted if tests A and D are carried out.
A. By IR: Determine by infrared absorption spectrophotometer. Compare the spectrum with that obtained with Dextromethorphan HBr RS.
B. When examined in the range 230nm to 360nm, a 0.01 per cent w/v solution in 0.1M hydrochloric acid shows an absorption maximum only at about 278nm.
C. In the test for related substances, the principal peak in the chromatogram obtained with test solution corresponds to that in the chromatogram obtained with reference solution.
D. Gives the reaction of bromides.
Reporting: Report as Complies/Does not comply.
Appearance of solution: A 5.0 per cent w/v solution in ethanol (95 per cent) is clear and colourless.
Reporting: Report as Complies/Does not comply.
Acidity or alkalinity: Dissolve0.4gm in carbon dioxide-free water with gentle heat, cool and dilute to 20ml with same solvent. Add 0.1ml of methyl red solution and 0.2ml of 0.01M sodium hydroxide. The solution is yellow and not more than 0.4ml of 0.01M hydrochloric acid is required to change the colour to red.
Reporting: Report as Complies/Does not comply.
Specific optical rotation: Determined in a 2.0 per cent w/v solution in 0.1 M hydrochloric acid.
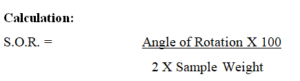
Reporting: Report as value in º.
N,N-Dimethylaniline: Dissolve 0.5g in 20ml water with the help of gentle heat on a water-bath, cool and add 2ml of 2M acetic acid, 1 ml of a 1 per cent w/v solution of sodium nitrite and sufficient water to produce 25 ml. The resulting solution is not more intensely coloured than that obtained by treating at the same time and in the same manner a solution containing 5µg of N, N-dimethylaniline in 20ml of water.
Reporting: Report as Complies / D
oes not comply.
Related Substances: Determine by liquid chromatography
Test Solution: Dissolve 10mg of the substance under examination in 10.0ml of the mobile phase.
Reference solution (a): A 0.0005 % w/v solution of dextromethorphan hydrobromide RS in the mobile phase. (Dissolve 50mg of the Dextromethorphan Hydrobromide RS in 100.0ml of the mobile phase. Take 1.0ml of this solution and dilute to 100ml with mobile phase).
Reference solution (a1): Precede same as reference solution (a).
Chromatographic system
– a stainless steel column 25cm x 4.6 mm packed with octadecylsilane bonded to porous silica (5µm),
– mobile phase: dissolve 3.11g of docusate sodium in a mixture of 400 ml of water and 600 ml of acetonitrile, Add 0.56g of ammonium nitrate, adjust the pH to 2.0 with glacial acetic acid,
– Flow rate. 1ml per minute
– spectrophotometer set at 280 nm
– Injection volume. 20µl.
Inject the test solution and the reference solution. Run the chromatogram twice the retention time of the principal peak. In the chromatogram obtained with the test solution the area of any secondary peak is not more than the area of the principal peak in the chromatogram obtained with reference solution (0.5per cent). The area of one such peak is not more than 0.5 times the area of the principal peak in the chromatogram obtain with reference solution.(0.25 per cent), and sum of all other secondary peaks is not more than twice the area of the principal peak in the chromatogram obtained with reference solution (1.0 per cent). Ignore any peak with an area less than 0.1 times the area of the principal peak in the chromatogram obtained with the reference solution .(0.05 per cent).

System suitability requirement:
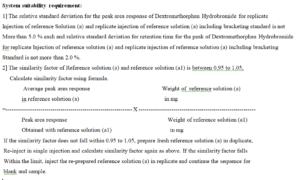
3] The column efficiency for the peak of Dextromethorphan Hydrobromide obtained in the chromatogram of individual
reference Solution (a) injected in replicates (including bracketing standard ) and reference solution (a) is not less then
2000 theoretical plates. (Report the value obtained from the fist injection of replicate of reference Solution (a) as well as
last bracketing standard).
Sulphated Ash: Weight accurately 1.0gm of sample in silica crucible ignites in muffle farness at 700ºC for 120 minutes.

DOCUMENT CHANGE HISTORY
| Revision No. | Changes |
| 00 | First Time Prepare |
| 01 | Periodic Revision and Review of Formating Standard Testing Procedure & Formate |
| 02 | Periodic Revision and Review of Formating Standard Testing Procedure & Formate |
| 03 | Periodic Revision change |
Analysis of vitamin B1 B6 B2 Nicotinamide and sodium pentothenate Injection
Analysis for Nandrolone Decanoate injection
Analysis of Dicyclomine and Diclofenac sodium Injection
standard testing procedure of Fexofenadine and Phenylephrine suspension
standard testing procedure of Piroxicam Injection
STP of Fungal Diastase and Papain capsules
standard testing procedure PVC
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
Analysis method of aceclofenac
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
Analysis method of Suspension of Tribasic Calcium phosphate with vitamin D3 and Vitamin B12
Analysis method of Calcitriol with calcium citrate Suspension
Analysis method of Oxyclozanide and Fenbendazole Suspension
Analysis method of Oxyclozanide and Levamisole Suspension
Analysis method of Triclabendazole and Ivermectin Suspension
Analysis method of Itraconazole Solution
Analysis method of Levocetirizine Dihydrochloride syrup
Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
Analysis method of Ferrous Ascorbate Cyanocobalamin and Folic Acid Suspension
Analysis method of Ambroxol Hydrochloride Drops
Analysis method of Ferrous Ascorbate with Folic Acid suspension
Analysis method of Piracetam Syrup
Analysis method of Rafoxanide and Levamisole suspension
Analysis method of Zinc gluconate Syrup
Analysis method of Magaldrate Simethicone and Oxetacaine suspension
Analysis method of mefenamic acid and paracetamol suspension
Analysis method of Cholecalciferol Drops
Analysis method of Racecadotril suspension
Analysis method of Deflazacort Suspension
Analysis method of Montelukast sodium and levocetirizine Dihydrochloride Syrup
Analysis method of Iron and Folic Acid Syrup
Analysis method of Cyproheptadine Hydrochloride and Tricholine Citrate Syrup
Analysis method of Levofloxacin Hemihydrate Ornidazole and Vitamin E Solution
Analysis method of Albendazole and ivermectin in oral liquid