standard testing procedure diclofenac sodium
Storage Requirements:
Store protected from light and moisture.
Sampling:
Sample equal quantity from each container / bag. Collect a minimum of 5g from each container/bag sample into individual, self –sealing clear polythene bag kept in another clear self sealing polythene bag bearing ‘Sample for Analysis,lable.After completion of sampling return rest sample on same container. Collect control sample in Pet Bottle/Glass Bottle.
Quantity of Composite Sample:
25 g
Quantity of individual Identification
0.0100 g from each and every container / bag.
Quantity of Control Sample:
2 X 25 g
Description: A white to slightly yellowish crystalline powder; slightly hygroscopic.
Solubility: Freely soluble in methanol; soluble in ethanol (95 per cent); sparingly soluble in water and in glacial acetic acid; practically insoluble in ether, in chloroform and in toluene.
Identification:
Test A may be omitted if tests B, C, and D are carried out. Tests B and C may be omitted if tests A and D are carried out
A. By IR.
B. A dark red colour develops.
C. The principal peak in the chromatogram obtained with the test solution corresponds to the peak in
The chromatogram obtained with the reference solution.
D. A 1.0 per cent w/v solution gives the reaction of sodium salts.
Appearance of solution: Not more intensely coloured than reference solution BYS6.
pH: 6.5 to 8.5
Light Absorption: Absorption at about 440nm NMT 0.050.
Related Substances: In the chromatogram obtained with the test solution; the area of any peak is not greater than the area of the principal peak is not greater than the area of the principal peak in the chromatogram obtained with the reference solution (0.2 per cent); the sum of the areas of all peaks other than the principal peak is not greater than 2.5 times that of the principal peak in the chromatogram obtained with the reference solution (0.5 per cent). Ignore any peak with an area less than 0.25 times the area of the principal peak in the chromatogram obtained with the reference solution.
Heavy Metals: NMT 10ppm.
Loss on drying: NMT 0.5%w/w.
Assay: 98.5% to 101.0%w/w on the dried basis.
Description: A white to slightly yellowish crystalline powder; slightly hygroscopic.
Solubility: Freely soluble in methanol
Soluble in ethanol (95 per cent)
Sparingly soluble in water and in glacial acetic acid
Practically insoluble in ether, in chloroform and in toluene.
Identification:
Test A may be omitted if tests B, C, and D are carried out. Tests B and C may be omitted if tests A and D are carried out
A. By IR.
B. To 1ml of a 0.4 per cent w/v solution in methanol add 1ml of nitric acid; a dark red colour develops.
C. In the test for related substances, the principal peak in the chromatogram obtained with the test solution corresponds to the peak in the chromatogram obtained with the reference solution.
D. A 1.0 per cent w/v solution gives the reaction of sodium salts.
Appearance of solution:
A 5.0 per cent w/v solution in methanol is clear and not more intensely coloured than reference solution BYS6.
pH: 6.5 to 8.5, deter mined on a 1.0 per cent w/v solution.
Light Absorption: Absorbance of a 5.0 per cent w/v solution in methanol at about 440nm, not more than 0.050.
Related Substances: Determine by liquid chromatography
Test solution: Dissolve 50mg of the substance under examination in methanol and dilute to 50ml with the same solvent.
Reference solution: A 0.0002 per cent w/v solution of diclofenac sodium RS in methanol.
Chromatographic system
– a stainless steel column 25cm X 4.6mm, packed with end-capped octylsilane bonded to porous silica (5µm),
– Mobile phase: a mixture of 34 volumes of a solution containing 0.5g per liter of phosphoric acid and 0.8g per liter of sodium dihydroen phosphate adjust to pH 2.5 with phosphoric acid, and 66 volumes of methanol.
– Flow rate. 1ml per minute,
– spectrophotometer set at 254nm,
– Injection volume. 20µl.
Inject the test solution and the reference solution. In the chromatogram obtained with the test solution: the area of any peak other than the principal peak is not greater than the area of the principal peak in the chromatogram obtained with the reference solution (0.2 per cent); the sum of the areas of all peaks other than the principal peak is not grater than 2.5 times that of the principal peak in the chromatogram obtained with the reference solution (0.5 per cent). Ignore any peak with an area less than 0.25 times the area of the principal peak in the chromatogram with reference solution.
Heavy Metals: 1.0 g complies with the limit test for heavy metals, Method B (10ppm).
Loss on drying: Not more than 0.5 per cent, determined on 1.0 g by drying in an oven at 105° for 3 hours.

Reporting: Report in %.
Assay:
Weigh accurately about 0.2g and dissolve in 50ml of anhydrous glacial acetic acid. Titrate with 0.1 M perchloric acid,
determining the end point potentiometrically.
Carry out a blank titration.
1 ml of 0.1 M perchloric acid is equivalent to 0.03181 g of C14H10Cl2NNaO2.
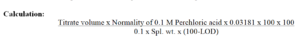
Reporting: Reporting as per %.
Analysis of vitamin B1 B6 B2 Nicotinamide and sodium pentothenate Injection
Analysis for Nandrolone Decanoate injection
Analysis of Dicyclomine and Diclofenac sodium Injection
standard testing procedure of Fexofenadine and Phenylephrine suspension
standard testing procedure of Piroxicam Injection
STP of Fungal Diastase and Papain capsules
standard testing procedure PVC
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
Analysis method of aceclofenac
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
Analysis method of Suspension of Tribasic Calcium phosphate with vitamin D3 and Vitamin B12
Analysis method of Calcitriol with calcium citrate Suspension
Analysis method of Oxyclozanide and Fenbendazole Suspension
Analysis method of Oxyclozanide and Levamisole Suspension
Analysis method of Triclabendazole and Ivermectin Suspension
Analysis method of Itraconazole Solution
Analysis method of Levocetirizine Dihydrochloride syrup
Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
Analysis method of Ferrous Ascorbate Cyanocobalamin and Folic Acid Suspension
Analysis method of Ambroxol Hydrochloride Drops
Analysis method of Ferrous Ascorbate with Folic Acid suspension
Analysis method of Piracetam Syrup
Analysis method of Rafoxanide and Levamisole suspension
Analysis method of Zinc gluconate Syrup
Analysis method of Magaldrate Simethicone and Oxetacaine suspension
Analysis method of mefenamic acid and paracetamol suspension
Analysis method of Cholecalciferol Drops
Analysis method of Racecadotril suspension
Analysis method of Deflazacort Suspension
Analysis method of Montelukast sodium and levocetirizine Dihydrochloride Syrup
Analysis method of Iron and Folic Acid Syrup
Analysis method of Cyproheptadine Hydrochloride and Tricholine Citrate Syrup
Analysis method of Levofloxacin Hemihydrate Ornidazole and Vitamin E Solution
Analysis method of Albendazole and ivermectin in oral liquid