General Test Procedure Identification by UV
- Purpose
- This procedure is applicable to all incoming Raw materials, In-process and Finished products.
- Apparatus
- UV-Visible spectrophotometer
- Quartz Cuvettes or Quartz Cells (1.0 cm)
- Reagents
- Freshly distilled Water
- Solvents as specified : AR Grade
- Chemicals as specified : AR Grade
- Procedure
- Set the spectrophotometer in the photometric mode. Select the specified wavelength. Prepare the test
solution as per the requirement by dissolving accurately weighed amount of sample in the specified
volume of solvent or diluents. Transfer the solvent or diluents, in which samples prepared, in both
the reference and sample cuvette.
-
- Place the cuvette in cuvette holders of the instrument. Auto zero the displayed reading of absorbance
to nullify the interference of the solvent. Remove only the sample cuvette from its holder, and rinse it
with about 2 to 3 ml of test solution, twice.
-
- Take UV absorption spectrum scan of sample and standard using 1 cm cells in the range 200 to 400
nm unless otherwise specified in the individual test procedure.
-
- The requirements are met if the Ultraviolet absorption spectrum of sample and standard exhibit
maxima and minima at the same wavelengths and absorptivities and or absorbance ratios are
within limit.
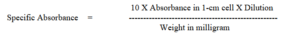
The Test solution must be clear. (Absorbance of hazy or partially dissolved substance cannot be measured.)
- Reference
- IP
- Revision History
-
Revision No.
Details of Changes
Reason for change
00
New GTP
NA
End of Document
- standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IPstandard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
analysis of Procyclidine HCl 5 mg Tablet
analysis of silodosin 8 mg capsule
analysis of Atorvastatin Calcium
General Test Procedure Identification by UV