Calibration Of Disintegration Test Apparatus
1.0 Objective
To lay down a guidelines for Calibration of Disintegration Test Apparatus in Quality Control Department.
2.0 Scope
This SOP applies to Calibration of Disintegration Test Apparatus used in Quality control Lab in A B C Company.
3.0 Responsibility
3.1 Executive – Quality Control.
3.2 In charge/Head.
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
QC : Quality Control
CC NO : Change Control Number
cGMP : Current Good Manufacturing Practice
C : Calibration
5.0 Procedure
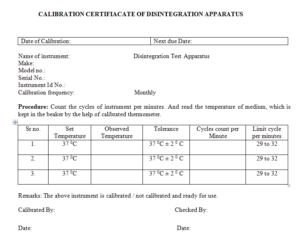
5.1 First check the instrument is free from dust and foreign matter.
5.2 Now on the Switch of main supply then on the switch of the instrument.
5.3 Fill the beaker with distilled water
5.4 Maintained the temperature in specified monograph.
5.5 Count the cycles of instrument per minutes and read the temperature of medium, Which is kept in the Beaker by the help of calibrated thermometer.
6.0 Forms and Records
6.1 Calibration Certificate of Disintegration Apparatus – Annexure-1
7.0 Distribution
7.1 Master copy – Documentation Cell (Quality Assurance)
7.2 Control copy – Quality Control
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
analysis of Procyclidine HCl 5 mg Tablet
analysis of silodosin 8 mg capsule
Method of Analysis Ceftriaxone and Sulbactam For Injection
Analysis of Glimepiride and Metformin Hydrochloride (SR) Tablets
General Test Procedure Melting Range and Freezing Point
General Test Procedure Weight per ML and Specific Gravity
General Test Procedure Distillation Range
General Test Procedure Non Volatile Matter and Residue on Evaporation
General Test Procedure Clarity of Solution
General Test Procedure Limit Test Chloride
General Test Procedure Limit Test Iron
General Test Procedure Limit Test Heavy Metals
General Test Procedure Water Soluble Substances
General Test Procedure Acid Soluble Substances
General Test Procedure 0.1M Ammonium Thiocyanate
General Test Procedure 0.1M Ethanolic Sodium Hydroxide
Calibration Of Disintegration Test Apparatus