analysis of pantoprazole and domperidone tablets
Each enteric coated tablet Contains:-
Domperidone IP 10 mg
Pantoprazole Sodium IP
Eq. to Pantoprazole 40 mg
Analysis Requirements:-
S. No. Test
1 Description
2 Identification
3 Average Weight
4 Uniformity of weight
5 Disintegration Time
6 Assay
ANALYSIS PROCEDURE
01. DESCRIPTION:-
Take the sample and keep in a well-lighted place. Observe physically its shape, colour texture, over printing etc.
02. IDENTIFICATION: –
In the assay the chromatogram obtained with test solution shows a peak having the same retention time as that of the peak due to Domperidone & Pantoprazole ws respectively.
03. AVERAGE WEIGHT:-
Weight Twenty (20) units taken randomly and record the total weight (Σ W). Divide the total weight by 20 to get the Average Weight.
Average Weight = Σ W ÷ 20
04. UNIFORMITY OF WEIGHT
Weigh twenty (20) units, taken individually and record their weights. Calculate the maximum and the minimum weights recorded. Not more than two of the individual weights deviate from the average weight by more than the percentage shown in the table and none deviates by more than twice that percentage.
| AVE. WT. OF TAB | PERMISSIBLE DEVIATION |
| Up to 80.0 mg | ± 10 % |
| More than 80.0 mg. but less than 250 mg. | ± 7.5 % |
| 250 mg. or more | ± 5.0 % |
05 DISINTEGRATION TIME :
Maintain temperature of the 0.1N Hydrochloric acid in the vessel of “Disintegration Test Apparatus” at 37.0oC ± 2.0oC and place one tablet in each of six tubes of the basket of apparatus; and operate the apparatus for 2 hour, no any tablet should be dissolve, break or crack .
Now replace the liquid with mixed Phosphate buffer Ph-6.8 (already maintain the temperature 37°C) add disc to each tube and operate the apparatus. Record the time within which all
the six tablets disintegrate completely and pass to the immersion fluid. All tablet should be disintegrate within 60 minutes.
If 1 or 2 tablets fail to disintegrate, repeat the test on 12 additional tablets; not less than 16 of the total of 18 tablets tested disintegrate.
If the tablets adhere to the disc and the preparation under examination fails to comply, repeat the test omitting the disc. The preparation complies with the test if all the tablets in the repeat test disintegrate.
- ASSAY:-
Buffer:- 680 mg Potassium dihydrogen dissolve in 100 ml Water,
adjust pH-6.8 with 1N
Sodium hydroxide
Mobile Phase : 60 ml Buffer & 40 ml Acetonitrile
Column : C18 (L1) 250×4.5mm
Flow rate : 1.0 ml/minute
Wavelength : 280 nm
Standard Preparation: –
(A) Weigh accurately 50 mg Domperidone WS in 50 ml volumetric flask, add 20 ml
Methanol heat to dissolve & dilute to 50 ml with methanol.
(B) To 10 ml solution A & 45 mg Pantoprazole Sodium WS +10 ml 1N Sodium hydroxide dilute to 50 ml with water.
(C) To 5 ml dilute to 25 ml with mobile phase.
Test Preparation: –
Weigh & powder of 20 tablets equivalent to 10 mg of Domperidone dissolve in 10 ml methanol heat to dissolve + 10 ml 1N Sodium hydroxide, cool and dilute to 50 ml with water.
To 5 ml dilute to 25 ml with mobile phase.
Procedure:
Separately inject 20 µl of standard and test solution. Record the chromatogram and measure the peak response of the measure peaks.
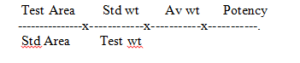
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
analysis of Albendazole Suspension
analysis of Acarbose 25 mg Tablet
Analysis of Memantine HCl 10mg Tablets
analysis of pantoprazole and domperidone tablets