standard test procedure ampicillin and cloxacillin capsules
Description VISUAL
EXAMINATION
IDENTIFICATION :- As per assay
Average weight & Weight variation (Content):
Equipment : A suitable analytical balance.
Procedure : Select 20 capsules from composite sample & weight accurately (a) . Now weight an intact capsule. Open the capsules without losing
any part of shell and remove the contents as completely as possible with the help of cotton plug. Weigh the shell. The weight of the contents is
the difference between the weighing (d). Repeat the procedure with a further 19 capsules. Find out total weight of 20 shells (b).
Deduct 20 weight of 20 shells (b) from weight of capsules (a). Calculate the average weight (content) (c) as follows

Disintegration Time:
Equipment: Disintegration test apparatus.
Procedure: Place 1 capsule in each of the six cylindrical tubes of the basket and operate the apparatus, using water maintained at 37±2° as
the immersion fluid. At the end of the 30 minutes, lift the basket from the fluid, and observe the capsules: all of the capsules have
disintegrated completely. If 1 or 2 capsules fill to disintegrate completely, repeat the test on 12 additional capsules: not less
than 16 of the total of 18 capsules tested disintegrate completely.
Assay
Each capsules on an average fill contain
Ampicillin Trihydrate IP
Calculate as Ampicillin
Cloxacillin Sodium IP
Calculate as Cloxacillin
Reagents
1. 0.3 N hydrochloric acid: Dilute12.7 ml of concentrate Hydrochloric acid to 500 ml with water
2. 1% v/v solution of formaldehyde in 0.3 N hydrochloric acid.Dilute12.5 ml of formaldehyde solution with sufficient 0.3 N hydrochloric acid to produce 500 ml
Standard Solution:
Accurately weigh quantity equivalent to 250mg of Cloxacillin and Ampicillin in a 500 ml
volumetric flask. Add 200 ml of water, sonicated for 15 minutes ensure complete dissolve &
makeup with water to 500ml, filter and use the filtrate for analysis.
Sample Solution
Accurately weigh quantity of the sample Capsule, equivalent to 250mg of the substance is suspended in 200 ml of water and sonicated for 15 minutes
ensure complete dissolve & dilute with water to 500ml, filter and use the filtrate for analysis.
Procedure for Cloxacillin :
Dilute 2ml each of the standard and sample solution to 50ml with formaldehyde reagent and allow to stand 20 minute. After 20 minute measure
the absorbance of sample and standard solution at 346 nm against reagent blank and calculate the contents of Cloxacillin in the sample by comparison with Cloxacillin sodium working standard
Calculation – Cloxacillin sodium calculated as Cloxacillin (mg / capsule)
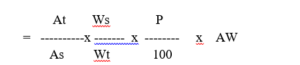
At and As are absorbance of the test preparation and standard preparation at 346 nm respectively.
Ws & Wt are dilution factor of the standard preparation and the test preparation respectively.
P is the percent purity of Cloxacillin sodium working standard as such basis
AW is the average weight of the capsule
Procedure for Ampicillin :
Dilute 2ml each of the standard and sample solution to 50 ml with formaldehyde reagent and heat of both the sample and standard solution at 90° C (±1°)
in constant temperature water-bath for 60minute, cool to room temperature and measure the absorbance at 380 nm for Amoxycillin against
reagent blank and calculate the contents by comparison with Ampicillin working standard
Calculation – Ampicillin trihydrate calculated as ampicillin ( mg / capsule )
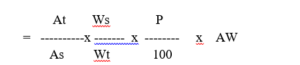
At and As are absorbance of the test preparation and standard preparation at 380 nm respectively.
Ws and Wt are dilution factor of the standard preparation and the test preparation respectively.
P is the percent purity of Ampicillin Trihydrate working standard as such basis
AW is the average weight of the capsule
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection