validation protocol for volumetric solution 0.1M ammonium Thiocyanate
1. Approval:
This document is prepared by the validation and the GMP compliance team
Under the authority of Quality Control Manager. Hence this document before being effective shall be
approved by QA team.
2. Purpose:
To establish documented evidence, on how the quality of a chemical substances or drug product varies with the time
under the influence of a variety of environmental factor such as Temperature, humidity and light during storage.
3. Scope:
This protocol provides a clear explanation of expectations when proposing a retest period or Shelf life and storage conditions
and outlines recommendations for establishing these from Single or multifactor and full reduced- design studies.
4. Reference :
Following documents are referred during preparation of the protocol
| Document Name | Document Number |
|
SOP for Volumetric Solution
|
|
5. Responsibility:
5.1 Execution:
Quality Control: Executive / Officer is responsible for analysis of physical and chemical Characteristic.
Manager QC: Verification & approval of results.

6. Validation Method:
VALIDATION OF VOLUMETRIC SOLUTION
PREPARATION AND STANDARDIZATION OF MOLAR SOLUTION
Name : ID. No .
STP No.:
Preparation:
Weigh accurately about 7.612g of Ammonium Thiocynate and transfer to a 1000ml volumetric flask. Dissolve in
sufficient purified water and make up to the mark with purified water.
OR
As per requirement (consumption history) prepare suitable amount of volumetric solution accordingly.
Standardization:
Pipette 30ml of 0.1M silver nitrate into a glass-stoperred flask, dilute with 50ml of purified water. Add 2ml of nitric acid
and 2ml of ferric ammonium sulphate solution and titrate with ammonium thiocynate solution until red-brown color appears.
Note down the volume of ammonium thiocynate solution consumed during titration. Repeat the procedure two more times.
Calculate the molarity of the solution by below given formula.
Each ml of 0.1M silver nitrate is equivalent to 0.007612g of NH4SCN
Determine the molarity in triplicate and calculate the average value where the variation is not more than 0.2%.
Preparation:
Weigh accurately about 7.612g of Ammonium Thiocynate and transfer to a 1000ml volumetric flask. Dissolve in sufficient
purified water and make up to the mark with purified water.
OR
As per requirement (consumption history) prepare suitable amount of volumetric solution accordingly.
Standardization:
Pipette 30ml of 0.1M silver nitrate into a glass-stoperred flask, dilute with 50ml of purified water. Add 2ml of nitric acid and
2ml of ferric ammonium sulphate solution and titrate with ammonium thiocynate solution until red-brown color appears.
Note down the volume of ammonium thiocynate solution consumed during titration. Repeat the procedure two more times.
Calculate the molarity of the solution by below given formula.
Each ml of 0.1M silver nitrate is equivalent to 0.007612g of NH4SCN
Validity: 30 day from the date of preparation and Re-Standardization after15 days.
Precaution – Check the physical appearance and fungal growth, sedimentation or change in color.
Storage: Store in glass bottles light-resistant containers. having well fitted, suitable stoppers.

7. Acceptance Criteria:
7.1 The volumetric solution of 0.1M Ammonium Thiocynate validity is 30 day from the date of preparation and
Re standardization every alternate days for validation and deviation should not be more then ±5.0%
7.2 Determine the molarity in triplicate and calculate the average value where the variation (precision) is not more
than 0.2%. This solution must be standardized frequently every month.
validation of volumetric solution molar solution calculation sheet
8. Result Reporting:
Calculation:
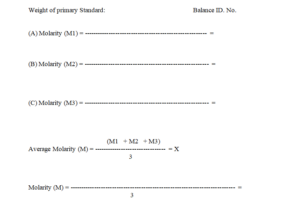
Variation (precision) is not more than 0.2%. =
9. Conclusion.
9.1 Normality of solution was found under acceptance criteria up to 35 days hence validity was define 30 days only
9.2 Solution shell be standardize every 15 days interval.
9.3 Solution must not be used after 30 days.
9.4 The validation exercise demonstrates that the Standardization of volumetric solution method of 0.1M Ammonium Thiocynate is stands validated.
sop for Calibration and Maintenance of Laboratory Instruments and Equipment
Disposal of Residual Sample or Left Over Material
sop for for Laboratory Incident
standard operating procedure temperature monitoring
sop for operation of infrared moisture balance
sop for preparation of mobile phase
sop for Preparation and Issuance of Analysis protocol standard
sop of placebo and impurity stock solutions
sop for disposal of residual sample
sop for handling of pharmacopoeial changes
sop for procedure for operation of ultrasonic cleaner
difference between UPLC and HPLC
sop for for Emergency Eyewash and Shower
sop for operation and calibration of total organic carbon analyzers
sop for operation of cobb tester
sop for Operation and calibration of atomic absorption spectrophotometer
sop for Operation and calibration of gas liquid chromatograph
sop for operation of humidity oven
sop for operation and calibration of serological water bath
sop for monitoring of drain trap
sop for destruction of analytical samples after testing and control samples
sop for destruction of used chemicals
Sop for Operation of suction pump
sop for Operation and calibration uv cabinet
sop for Operation and calibration of bulk density apparatus
sop for operation and calibration of shore hardness tester
sop for operation of rub proofness tester
sop for monitoring of purified water
sop for Retesting of packaging materials
sop for Retesting and resampling of raw materials
sop for Control of issuance of record of analysis green sheets
sop for Control of computer passwords
sop for sampling of packaging materials PM
sop for sampling of sterile raw material
sop for sampling of intermediates and finished products
sop for operation and calibration of friability test apparatus
sop for approval and rejection of packaging materials
sop for non conformance of RM PM and finished product
sop for collection storage and disposal of control samples
sop for trend analysis of finished products
sop for Chromatographic practices and system suitability
SOP For Good Laboratory Practices
sop for cleaning and operation of sieve shaker
general specification of packing material cartons
sop for Password for Analytical Instrument and LIMS software
sop for Rounding off numerical analytical results
sop for sampling of bulk and finished product
sop for cleaning of spillage material
sop for Handling of Reference Standard
sop for hplc column maintenance and washing
procedure for sampling and handling of bulk sample
STP for borewell generation point (raw water storage tank)
sop for preparation and standardization of 0.1M Zinc Sulphate
Operation &calibration of analytical balance (dhona)
Operation and Calibration Procedure for Disintegration Test apparatus
sop for preparation and standardization of 1 M Hydrochloric Acid
Preparation and standardization of 0.1 M ceric ammonium sulphate solution
sop for preparation and standardization of 0.05 m iodine solution
validation of volumetric solution 0.1m ammonium thiocyanate
handling of reference standard and preparation of working standard
sop for water sampling and analysis
sop for operation for validation of excel worksheets
sop for stability of volumetric solutions
sop for preparation of raw material in process finish product packing material data sheets
sop for handling of hazardous chemicals
sop for handling of glassware and allocation of identification number
sop for operation cleaning and calibration of bursting strength tester
sop for rounding off the analytical test results
procedure for Analyst Qualification
sop for operation and calibration of dissolution Apparatus
procedure for maintenance of desiccators
sop for for hplc column receipt checking id no and regeneration
safety data sheet for laboratory chemicals
procedure for handling of poisonous chemicals
sop for cleaning of sampling devices
sop for calibration procedure of instruments
sop for specification and standard testing raw material packing material and finished product
procedure for operation and calibration of potentiometric titrator
procedure for operating and calibration of digital hardness tester
procedure for disposal of expired chemicals, reagents and solvents
sop for behavior in quality control department
sop for preparation and standardization 0.1M sodium thiosulphate
sop for preparation and standardization 0.1M Disodium Edetate
preparation and standardization 0.1M Sodium Hydroxide Solution
Preparation and standardization of 0.1M Perchloric acid solution
sop for preparation 0.05M edetate disodium
sop for preparation 0.1M silver nitrate
sop for Operation and Calibration of High Performance Liquid Chromatography
sop for UV & Visible Spectrophotometer
procedure for Cleaning of laboratory glassware
Cleaning of Instrument, Instrument bench and surrounding area of Quality
Safety Precaution in Quality Control Department
Operation & Calibration of Analytical Balance
Calibration of Glassware in Quality Control Department
handling of samples received in Quality Control
Cleaning and Operation of Refrigerator
Operation, Cleaning and Calibration of water bath
Operation & Calibration of Refractometer
Operation and Cleaning of Centrifuge Apparatus
cleaning, operation & calibration of Vernier caliper
Calibration of Fourier Transform Infrared Spectrophotometer (FTIR)
Cleaning and operation of Moisture Analyzer
Cleaning & Operation of Vacuum pump in Quality Control Department
Operation and Calibration of Polarimeter
Cleaning and operation of Magnetic Stirrer
Cleaning Operation and Calibration of Melting Point
Operation Cleaning and Calibration of Muffle Furnance in Quality Control Department
procedure of operation and Cleaning of Sonicator
Operation Cleaning & Calibration of pH meter in Quality Control Department
Entry and Exit in Quality Control Department