microbiological assay of cyanocobalamin or vitamin B12
1.0 OBJECTIVE
To describe the procedure for determine the assay of Cyanocobalamin or vitamin b-12 by biological method using E. Coli mutant MTCC -452.
2.0 PURPOSE
It is the policy of (Analytical Division) that a written procedure shall be followed for determine the assay of Cyanocobalamin or vitamin b-12 by biological method using E. Coli mutant MTCC -452.
3.0 SCOPE
This SOP shall be applicable for in Microbiology Laboratory
4.0 RESPONSIBILITY
Persons along with their responsibilities are given below
| Sr. No | Designation | Responsibility |
| 01 | Microbiologist | Preparation & revision of SOP. |
| 02 | Officer-QA | Training, Distribution and retrieval of SOP. |
| 03 | Quality Manager | Approval & Implementation of SOP. |
5.0 PROCEDURE
5.1 Method : Cup plate method
5.2 Culture maintenance medium : B-12 culture agar (E.coli maintenance medium) M-185
5.3 B12 Assay Agar Himedia M 110
5.4 Assay Medium :
| Sr. No | Ingredients | Quantity / 100 ml. |
| 1 | Dipotassium Phosphate | 1.29 g |
| 3 | Anhydrous Citric acid A.R. | 1.1 g |
| 4 | Sodium metabisulphate A.R | 1.0 g |
| 7 | Distilled Water | 100 ml |
Dissolve the ingredients separately to avoid precipitation & shake and mix the solution.
If necessary, adjust the pH of the solution to 7.0 ± 0.1. Sterilize at 15 lbs pressure for 20 minutes.
5.5 Inoculum medium : Buffer Peptone Water
Adjust the pH of the solution to 7.0 ± 0.1. Dispense in screw capped test tubes and autoclave at 15 lbs pressure for 15 minutes. Cool & store at 4OC.
5.6 Preparation of Culture Suspension:
Streak the culture on B-12 maintenance agar slant (Himedia M 185) and on the day use inoculate loop full culture in 20 ml of peptone water and incubate 30-35 ºC for 3-4 hours.
5.7 preparation of assay plates:
Vitamin B12 assay medium (M110) is sterilized at 121ºC for 25 minutes ,and cooled to 40-45ºC, Add 3-4 ml of inoculum of E.coli mutant MTCC 452 of required thickness. Mix gently but thoroughly. Distribute 25 to 30 ml of the inoculated medium in sterile petri plates. Allow to set the medium & store in refrigerator until use. Plates should be used on the same day or within one day.
5.8 Preparation of Agar Cups :
A standard 8.0 mm diameter borer is taken. Dip the borer in Isopropyl alcohol and burn the remaining Isopropyl alcohol from borer on flame, cool the borer properly and bore cups in preseeded agar plates. Bore four cups per plates.
5.9 Preparation of Standard Stock Solution :
Weigh accurately 25 mg of crystalline Cyanocobalmin powder (dark red coloured). Transfer it to 1000 ml volumetric flask and make up the volume to 1000 ml (A). Take the reading of this solution at 361 nm and calculate the conc. taking E 1 % as 0.207. Calculate exactly the conc. of vitamin B12/ ml .Take 2 ml and dilute it to 200 ml (A) with water. Take 2 ml (A) and dilute it to 20 ml (B). Take 10 ml (B) and dilute it to 20 ml(C) with water.
5.10 Calculation :
O.D. at 361
————– X 100 = X mcg/ml.
0.207
Accordingly calculate X ml [about 10 ml of this solution] dilute to 100 ml to get [1 mcg/ml]. This is one mcg /ml stock, use within one month.
6.0 Preparation of Standard Dilutions :Preparation of Test Dilution : Weigh and transfer a sample quantity eq. to 5 mcg. to 50 ml volumetric flask. Add distilled water, shake vigorously and dilute to 50 ml with distilled Water(TH). Further dilute 5 ml of the TH to 50 ml with distilled water[TL]. Application of standard and Test Dilutions : With the help sterile micropipette tips apply 100 µl of different dilution to different cups. Mark every cup with proper dilution and keep the plates at low temperature (around 10°C) for 10-20 minutes for diffusion.
6.1 Protocol Of Standard Dilution:
25 mg—————————–1000 ml (Stock solution)
A) 2 ml(Stock solution)—————200 ml
B) 2 ml (A)———————————-20 ml (Standard Higher)
C) 10 ml (B)——————————–20 ml (Standard Lower)
6.2 Incubation of Plates :
Incubate the plates at 30-37°C for overnight (18-24 hours).
6.3 Measurement of Diameter :
Measure the diameter of every zone of growth from three different sides. Note down every reading.
6.4 Calculations :
Sum up three readings of individual dilution.
Formula –
Percent potency (%P) = Antilog (2 + a log I) Where,
(TH + TL) – (SH + SL)
a = ———————————–
(TH – TL) + (SH – SL)
6.5 Ratio of Dilution = I = 1 : 2
log 2 = 0.3010
Calculations :
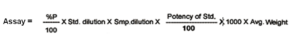
6.6 Protocol of Dilutions :
A 25 mg ——> 1000 ml
B 2 ml(A) ——-> 200ml
SH 2ml (B) ——> 20 ml 0.25 mcg/ml
SL 10 ml (Stock) ———-> 20 ml 0.125 mcg/ml
7.0 ACCEPTANCE LIMIT: Not less than 90 percent.
8.0 ABBREVIATIONS & DEFINITION
QA Quality Assurance
SOP Standard Operating Procedure
QM Quality Microbiology
FR Format
S. No. Serial Number
ml Milliliter
mcg microgram
°C Degree centigrade

sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware