analysis of Albendazole Suspension
standard testing procedure albendazole Suspension
1.0 OBJECTIVE
1.1 To lay down a procedure for analysis of Albendazole Suspension.
2.0 SCOPE
2.1 This procedure is applicable to the analysis of Albendazole Suspension. in QC lab at abc pharma.
3.0 RESPONSIBILITY
3.1 Q.C- Chemist
4.0 ACCOUNTABILITY
4.1 Manager-Quality Assurance
5.1 Description: Pour 50ml finish sample in beaker and observed visually.
5.2 pH: Taken 50 ml sample in beaker rinse the pH electrode first with purified water followed by sample dip the electrode in sample and observed the pH.
5.3 Volume variation: Measured the volume by measuring cylinder and determine the volume variation.
5.4 Identification:
5.4.1 In the assay, the principle peak in the chromatogram obtain with the test solution correspond to the peak in the chromatogram obtained obtained with the reference solution.
5.5 ASSAY:
Each 5 ml contains
Albendazole I.P. 200 mg.
Releted substance: -Determine by liquid chromatography
Note; Prepare the solution immediately before use.
Solvent mixture; 30 volumes of 0.015M ammonium dihydrogen orthophosphate and 70 volumes of methanol.
Test Solution. dilute a quantity of the oral sups. Containing 500mg of Albendazole to 50.0ml with 1.0 percent w/v solution of Methanolic sulphuric acid dilute 5.0ml of this solution to 10.0ml with the solvent mixture.
Reference solution (a) Dilute 1.0ml of the test solution to 100.0ml with the solvent mixture.
Reference solution (b) Dissolve 25mg each of Albendazole RS and oxibendazole RS in 5.0ml of 1.0 percent v/v solution of Methanolic sulphuric acid and dilute to 50.0ml with solvent mixture.
Chromatographic system; a stainless steel column 25cm x4.6mm packed with octadecylsilane bonded to porous silica (5um)
Mobile phase A.0.015M ammonium dihydrogen orthophosphate in water.
Methanol; a gradient programme using the condition given flowrate 0.7ml per minute
Spectrophotometer set at 292nm
Injection volume 20ul

Resolution between two principal peak is not less than 7.0
Method of Albendazole.
Mobile phase: – A. 0.015M ammonium dihydrogen orthophosphate in water.
Diluent: – Methanolic sulphuric acid.
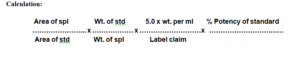
Standard Preparation: Taking weight 100 mg standard. of Albendazole diluted to 100 ml Methanolic sulphuric acid. Further dilution 5.0 ml to 25 ml with Methanolic sulphuric acid.
Sample preparation: weight accurately 100mg sample wt. of Albendazole. diluted to 100 ml with Methanolic sulphuric acid. Further dilution 5.0 ml to 25 ml with Methanolic sulphuric acid.
Chromatographic system: – column C18 (25cm x4.6mm) porous silica (5um)
Wavelength -292 nm
Flow rate- 0.7 ml/mint.
C18 (250 x4.6) 5 micron
Injection volume 20 µl
 \
\
6.0 ABREVIATIONS
| Sr. No. | Abbreviation used | Full form of abbreviation used |
| 1.0 | STP | Standard Testing Procedure |
| 2.0 | QA | Quality assurance |
| 3.0 | STD | Standard |
| 4.0 | SPL | Sample |
| 5.0 | NM | Nano Meter |
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
analysis of Albendazole Suspension