Handling Of Dispensed Material
1.0 Objective
The purpose of this procedure is to describe the handling of raw and packaging materials in manufacturing.
2.0 Scope
This Standard Operating Procedure is applicable for covers handling of dispensed raw and packing
material, additional material requisition, material return and on line rejection of any material at A B C Company.
3.0 Responsibility
3.1 Production Executive
3.2 Operator
3.3 Head – Quality Assurance
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure
QA : Quality Assurance
IPQA : In process quality Assurance
5.0 Procedure
5.1 Handling of dispensed raw material:
5.1.1 Shift the dispensed raw material from the dispensing room to the day store room.
5.1.2 Ensure that all the material dispensed for that batch has been shifted.
5.1.3 Ensure that all the items are labeled properly.
5.1.4 Keep all the dispensed raw material of the batch together.
5.1.5 Never keep raw material of two different batches on the same pallet/cage or nearby.
There should be some distance between the two batches and proper segregation.
5.1.6 Whenever the batch is planned, open the day store room and transfer the raw material to the process area.
5.1.7 Ensure that the raw material for the batch planned is being transferred. Get it verified by
the Production Officer for batch identity.
5.1.8 Lock the day store room door.
5.1.9 Production chemist shall check individual raw material of the batch for batch identity before
starting with the processing activity.
Note: Keep the day store room under lock and key. Open it in the presence of Production chemist only.
5.2 Handling of dispensed packing material:
5.2.1 Handling of Primary Packing Material
5.2.1.1 Ensure that packing materials, to be dispensed, are released by Quality Control.
5.2.1.2 Ensure that the entire primary packing materials dispensed, as per Packing Material
Requisition, is properly labelled and segregated.
5.2.1.3 Transfer dispensed primary packing material, in the ‘Foil/PVC Staging Area’ in Process Area through pass box.
5.2.1.4 Keep the printed primary packing material under lock and key.
5.2.1.5 During packing of the batch, sample of each lidding foil roll should be checked for its code number
and attach the roll sample in the BPR.
5.2.1.6 Load the lidding foil and base film roll as per the QC Reference Number mentioned in the Material
Requisition; following the FIFO system (in case two batch of same material is dispensed).
5.2.1.7 At the end of the batch, return extra material to PMS on Material Return Note.
5.2.2 Handling of Secondary Packing Material
5.2.2.1 Ensure that the entire secondary packing materials dispensed, as per Packing Material
Requisition, is properly labeled and segregated.
5.2.2.2 Keep dispensed secondary packing material, in the ‘Packing Hall’.
5.2.2.3 Keep the printed secondary packing material under lock and key.
5.2.2.4 During packing of the batch, sample of each printed aluminium foil should be checked for it’s code
number and attach the sample in the BPR.
5.2.2.5 Consume all the packing material as per the QC Reference Number mentioned in the Material
Requisition; following the FIFO system (in case two batch of same material is dispensed).
5.2.2.6 At the end of the batch, return extra material to PMS on Material Return Note.
5.2.2.7 Handling of Overprinted Packing Material.
5.2.2.8 Before starting of overprinting activity, clean the entire machine.
5.2.2.9 Take the line clearance of the machine and room from the Production and In Process Quality Assurance chemist.
5.2.2.10 After line clearance, take the material inside the overprinting room.
5.2.2.11 Take a proof of the overprinted item and get it verified by the Production and In Process
Quality Assurance chemist. Attach this proof to BPR.
5.2.2.12 Start the overprinting activity after certification of the first proof.
5.2.2.13 Carry out overprinting operation of only one batch of a product at a time and collect in a pre
labelled crate/cage as ‘Over Printed Material to Be Checked’.
5.2.2.14 Check the overprinted materials and keep it directly in pre labelled crate/cage,
as ‘Over printed Material Checked’, provided for the batch.
5.2.2.15 If the overprinting activity lasts for more than a day, at the end of the shift, keep the
overprinted and to be overprinted material segregated under lock and key in the cage.
5.2.2.16 After completion of overprinting activity attach last sample to the BPR after checking by Production chemist.
5.2.2.17 Reconcile the packing material as per the BPR.
5.2.2.18 Attach a specimen sample of the overprinted material to the BPR if it is carried out on next day also and so on.
5.3 Additional Material Requisition:
5.3.1 Additional raw materials shall be required in the conditions of on line rejection and spillage
of any material as well as for the performance trial of any equipment with authorization of quality and production head.
5.3.2 Additional packing materials shall be required in the conditions of on line rejection and
shortage of any material as well as for the performance trial of any equipment with
authorization of quality and production head.
5.3.3 Fill the Additional Material Requirement Form with Product, Batch No., Date,
Market, Batch Size, Manufacturing Date, Expiry Date, Product Code, Stage, Material Required, Code No., Quantity and Reason.
5.3.4 Get the duly signed form verified from QA chemist and Manager Production.
5.3.5 QA person has to write the comment (if any).
5.3.6 The verified form shall be evaluated and authorized by the Manager QA and GM Plant.
5.3.7 Send the form to ware house for dispensing.
5.4 Material Return:
5.4.1 Packing materials can be returned back to the warehouse under the following conditions.
5.4.2 At the end of the packing operation certain packaging components like label, Printed and
plain aluminum foils, PVC, carton etc could be remaining and no further batch is there for packing.
5.4.3 If there is a major breakdown of the equipment and the packing of that batch needs to be cancelled.
5.4.4 If there is a sudden revision in the packaging component and the batch has to be packed with the
new packaging components.
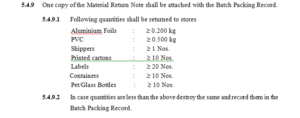
5.4.5 Keep all the remaining un-coded packing material that is to be sent on Material Return Note to
Warehouse in the cage under lock and key after batch completion.
5.4.6 Complete the Material Return Note with Date, Material Code No., QC Reference No., Quantity and
get it verified from IPQA chemist.
5.4.7 Count /weigh the materials and keep the packaging components in polybag with proper status label.
5.4.8 Hand over the material to Warehouse.
6.0 Forms and Records
6.1 Additional Material Requisition – Annexure-1
6.2 Material Return Note – Annexure-2
7.0 Distribution
7.1 Master Copy – Documentation Cell (Quality Assurance)
7.2 Controlled Copy – Production Department
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |
Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt
sop for Cleaning and operation of spray gun and assemble
sop for Fogging in Aseptic and Non Aseptic Area
Cleaning And Assembly Of Tube Filling Machine
Proper Handling of Non Recoverable Residues
sop for Handling Of Off Line Packing