sop for Issuance retrieval and destruction of BMR and analytical records
1.0 OBJECTIVE
The objective of this SOP is:
1.1 To lay down a procedure for issuance, Storage, Retrieval and Destruction of BMR and Control Records.
2.0 RESPONSIBILITY
2.1 Officer / Executive – Quality Assurance (QA) shall be:
2.1.1. Responsible for issuing a current and approved copy of BMR with respect to BMR No., Batch No, Batch size, Manufacturing Date and Expiry Date.
2.1.2. Responsible for making entries with respect to BMR No. Batch Size, Issue Date, Mfg. Date, Exp. Date, Price in BMR Issuance Register.
2.1.3. Responsible for receipt and storage of completed BMRs & Control Records in the documentation cell for a specified period.
2.2 Officer –Production / Warehouse
2.2.1 Responsible for intimation for issuance of BMR to Quality Assurance, Receipt of BMR & submission of completed BMR on batch completion to Quality Assurance.
3.0. ACCOUNTABILITY:
Head – Quality Assurance
4.0. PROCEDURE:
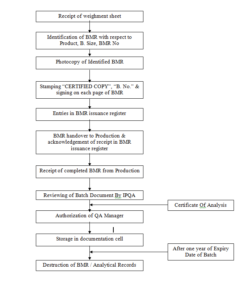
4.1 PROCEDURE FOR ISSUANCE:
4.1.1. On receiving weighment sheet, Officer – QA will issue the BMR.
4.1.2. The Photocopy of Master Copy of BMR shall be issued by stamping “CERTIFIED COPY” on each page of BMR at the right side bottom corner.
4.1.3. Certified copy shall be signed by QA- Officer and Batch Number shall be written on each page of BMR in green ink.
4.1.4. Officer – QA shall record the issuance of BMR in BMR Issuance Register (Refer Annexure– 1)
4.1.5. The Certified copy of BMR shall submit to Production and acknowledgement shall be taken in the BMR Issuance Register.
4.2 PROCEDURE FOR STORAGE
4.2.1 Completed BMRs & analytical Records shall be stored in documentation cell after ensuring that BMR is complete in all aspects as per SOP No. A/QA/005.
4.2.2 BMRs shall be stored for the period of one year from the date of expiration of the product.
4.2.3 Analytical records shall be stored for one year from the date of expiration of the product.
4.3 RETRIEVAL OF BMRs FROM DOCUMENTATION CELL
4.3.1 BMRs from documentation cell can be issued for review or study only with written permission of Quality Assurance Manager or Quality Control Manager.
4.4 DESTRUCTION OF BMRs
4.4.1 All BMRs and analytical records due for destruction shall be taken out from documentation cell and taken to shredder.
4.4.2 All these documents shall be disposed off by shredding in shredder in presence of Quality Assurance – Officer.
5.0 TRAINING:
Trainer — Head – Quality Assurance
Trainees — Executive / Officers QA / Production / Warehouse
6.0. DISTRIBUTION:
Certified Copy : Head – Plant Operation
Original Copy : Head – QUALITY ASSURANCE.
7.0 ANNEXURES:
Annexure – 1 : Format for BMR Issuance Register
Annexure – 2 : Schematic Diagram
8.0 REFERENCE:
In-house
ANNEXURE – 1
FORMAT FOR BMR ISSUANCE REGISTER
BMR / BMR ISSUANCE REGISTER
Product Name: Page No.:
Sr. No. |
Date |
Batch No. |
BMR / BMR No. |
Batch Size |
Price |
Mfg. Date |
Expiry Date |
Issued By |
Received By |
Remark |
ANNEXURE – 2
SCHEMATIC DIAGRAM
Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt