Analyses of Mesalamine Delayed Release Tablets
TEST PROCEDURE / METHODS
Standard testing procedure of Mesalamine Delayed Release Tablets
01. Description: Remove 20 tablets from the strip. Place on a white paper and observe visually.
02. Average Weight: Weigh accurately 20 tablets and calculate the average weight.
Average Weight = Weight of 20 tablets / 20
03. Uniformity of weight: variation Weigh 20 tablets selected at random and determine the average weight. Weigh 20 tablets individually & record. Not more than two of the individual weights deviate from the average weight by more than 5 % and none deviates by more than 10 %.
04. Identification
In the Assay, the peak maxima in the chromatogram obtained with the test solution corresponds to the peak maxima due to misalamine in the chromatogram obtained with the reference solution due to scanning on UV & Vis Spectrophotometer.
05 Leak test Equipment:
a) A vacuum Desiccator.
b) A vacuum pump with gauge.
Procedure: Take 3 strips and keep in vacuum desiccator. Ensure that strips are immersed in water. Connect the desiccator to vacuum pump with rubber tube and apply vacuum about 381 mm (15”) of mercury. The vacuum should be maintained for 30 sec. Release vacuum. Remove the strips wipe and dry with a clean cloth cut the strip and examine carefully. None of the tablets should be sticky or moist.
06 Dissolution :Test Acid-Stage
Apparatus No. 1(Paddle)
Medium. 500 ml of 0.1HCl,
Speed and time. 100 rpm for 120 minutes.
Withdraw a suitable volume of the medium and filter. Determine by UV & Vis Spectrophotometer
Test solution. Use the filtrate.
Reference solution. A 0.002 per cent w/v solution of Mesalamine WS in dissolution medium.
Use UV & Vis Spectrophotometer system to take the absorbance at 302nm.
Calculate the content of Mesalamine.
D. Not more than 10 per cent of the stated amount of Mesalamine.
Buffer-Stage-I
Apparatus No. 1, (Paddle)
Medium. 900 ml of phosphate buffer pH-6.0 (Prepare by using 42.3gm of KH2PO4 & 1.65gm NaOH to 2litter Volumetric Flask)
Speed and time. 100 rpm for 60 minutes.
Run the dissolution with tablet used in the acid-stage after drying with tissue paper, Withdraw 50ml aliquot and filter, Determine by UV & Vis Spectrophotometer immediately.
Test solution. Use the filtrate.
Reference solution. A 0.002 per cent w/v solution of Mesalamine WS in dissolution medium.
Use UV & Vis Spectrophotometer system to take the absorbance at 330nm.
Calculate the content of Mesalamine.
D. Not more than 10 per cent of the stated amount of Mesalamine.
Buffer-Stage-II
Sodium Hydroxide Solution: 133.6gm NaOH to a 2 liter VF & dilute to water.
Apparatus No. 1, (Paddle)
Medium: As such medium used in buffer stage-I by adding 50ml of Sodium Hydroxide Solution & check the pH-7.2.
Speed and time. 50 rpm for 90 minutes.
Run the dissolution with tablet used in the buffer stage-I, Withdraw a suitable volume of the medium and filter. Determine by UV & Vis Spectrophotometer
Test solution. Use the filtrate.
Reference solution. A 0.002 per cent w/v solution of Mesalamine WS in dissolution medium.
Use UV & Vis Spectrophotometer system to take the absorbance at 332nm.
Calculate the content of Mesalamine.
D. Not less than 80 per cent of the stated amount of Mesalamine.
7.0 Assay :
Mesalamine-400mg Estimation of Mesalamine By UV Spectrophotometer
Standard Preparation: Weight Accurately 100mg WS of Mesalamine in 100ml of VF dissolve in 70ml of 0.1N NaOH and dilute to 100 ml with 0.1N NaOH further dilute 2ml in 100 ml of VF and make up to 100ml with 0.1N HCl.
Test Preparation: Cruses The 20 Tablets Weight Accurately Eq. to 50mg of Mesalamine in 100ml of VF sonicate to dissolve in 70ml of 0.1N NaOH and dilute to 100ml with 0.1N NaOH further dilute 2ml in 100ml of VF and make up to 100ml with 0.1N HCl.
And measure the absorbance by UV & Vis Spectrophotometer at 302 nm and calculate the results by comparison.
Sample Std Wt.
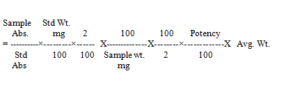
(Calculate the result of labeled amount of Mesalamine, Since the labeled amount of Mesalamine is 400mg
Abbreviations:
WS :- working standard
VF :-Volumetric flask
STD :-Standard
Abs. :-Absorbance
Spl. :-Sample
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
analysis of Albendazole Suspension