Analysis of Levocetirizine Tablets
TESTS TEST PROCEDURE / METHODS
01. Description Remove 20 tablets from the strip. Place on a white paper and observe visually.
02. Identification
In the assay, the principle peak in the chromatogram obtained with the test solution corresponds to the peak in the chromatogram obtained with reference solution.
03. Average Weight: Weigh accurately 20 tablets and calculate the average weight.
Average Weight = Weight of 20 tablets / 20
04. Uniformity of Content: Use the chromatographic condition of assay to perform the Uniformity of content.
Standard Preparation
As described in the assay
Test Preparation
1 tablet in 100 ml of Volumetric Flask dissolve in 50 ml of mobile phase heat upto 45°C, sonicate for 1 minute and dilute to 100 ml with mobile phase.
Procedure
As described in the assay
Calculation
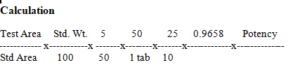
05 Dissolution Apparatus No. 1, Paddle
Medium. 900 ml of phosphate buffer pH 6.8,
(40.8 gm KH2PO4 + 5.4 gm Sodium hydroxide dissolve in 6.0 liters water).
Speed and time- 50 rpm for 30 minutes.
Withdraw a suitable volume of the medium and filter. Determine by HPLC Spectrophotometer
Standard Preparation: Weight Accurately about 50 mg WS of levocetirizine dihydrochloride in 100 ml of Volumetric Flask dissolve in 50 ml of mobile phase heat upto 45°C, sonicate for 1 minute and dilute to 100 ml with mobile phase.
To 2 ml dilute to 200 ml with dissolution medium.
(Keep at 37°C at least for 30 minutes)
D. Not less than 75%.
Procedure
As described in the assay
Assay Buffer – 680 mg Potassium dihydrogen phosphate dilute to 100 ml with water
Mobile phase-60V Buffer+40V Acetonitrile adjust pH-6.0 with 1N Sodium hydroxide.
Sodium Hydroxide- 40 gm Sodium hydroxide dissolve in 1000 ml water.
Column- A stainless steel column 250 x 4.6 mm to porous silica
Flow rate : 1 ml per minute
Wavelength : 230 nm
Injection volume: 20 µL
Standard Preparation: Weight Accurately about 50 mg WS of levocetirizine dihydrochloride in 100 ml of Volumetric Flask dissolve in 50 ml of mobile phase heat upto 45°C, sonicate for 1 minute and dilute to 100 ml with mobile phase.
To 5 ml dilute to 50 ml with mobile phase.
Test Preparation: weight & powder 20 Tablets weight Accurately Eq. to 25 mg of levocetirizine dihydrochloride in 100 ml of Volumetric Flask dissolve in 50 ml of mobile phase heat upto 45°C, sonicate for 1 minute and dilute to 100 ml with mobile phase.
To 5 ml dilute to 25 ml with mobile phase.
Calculation
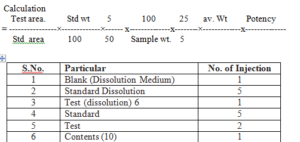
Abbreviations:
WS- working standard
VF-Volumetric flask
STD-Standard
Spl.-Sample
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
analysis of Albendazole Suspension