sop for calibration and verification of check weigher
1.0 OBJECTIVE:
To lay down a procedure for Operation and Cleaning of Checkweigher.
2.0 SCOPE:
This SOP is applicable for Operation and Cleaning of Checkweigher in Three Piece Line
3.0 RESPONSIBILITY:
Officer/Executive – Production
4.0 ACCOUNTABILITY:
Head – Production
5.0 PROCEDURE:
5.1 INSTRUCTIONS:
5.1.1 Check the cleanliness of the Checkweigher and ensure that is free from remains of the previous product.
5.1.2 Speed of Checkweigher NMT 120 vials/min.
5.1.3 Speed of infeed star wheel NMT 6.5 round/min.
5.2 OPERATION:
5.2.1 Switch “ON” the Main Switch.
5.2.2 Turn the power switch 90 degrees clockwise to the “ON” position.
5.2.3 Touch the ‘LOGIN’ icon and make the entry of ‘LOGIN ID’ and ‘LOGGIN PASSWORD’ then click on ENTER.
5.2.4 After the successful login, Touch to ‘MENU’ icon to open the Product Selection screen.
5.2.5 Touch the “PRODUCT SET UP” then touch the “PRODUCT DATA” for Edit icon to register the product and open the Product Setting Screen.
5.2.5.1 Touch to unregistered icon and make sure the entry of Product Name and Product Code.
5.2.5.2 After the entry of name and code to save Re login the login ID and login password
5.2.5.2.1 Select target to enter the mass of the product to be weighed.
5.2.5.2.2 Select high limit to enter the upper limit mass value of the product.
6.2.5.2.3 Select lower limit to enter the lower limit mass value of the product.
6.2.5.2.4 Touch the “NEXT PAGE” key to return to the Product Display screen.
5.2.5.2.5 Record the Operation details in Format No Titled “Equipment Log”
5.3 CHALLENGE TEST OF CHECKWEIGHER:
5.3.1 Prepare the lower & higher weight vials for challenge test from the respective batch i.e. lower weight vial shall be prepared by removing the solution from vial upto below lower limit & higher weight vial shall be prepared by poring the solution in another vial above from the higher limit.
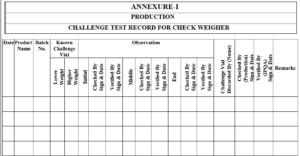
5.3.2 Pass these vials from the Checkweigher before start the batch, middle of batch & end of batch, record the observation in Annexure-I, Titled “Challenge Test Record for Checkweigher”.
5.3.3 If challenge test passed start the weighing of filled vials by Checkweigher and if challenge test failed inform to the Engineering department for the rectification of Checkweigher.
5.3.4 After rectification of Checkweigher again perform the challenge test as per section no. 5.3.1
and 5.3.2.
5.3.5 At the end of process challenge test vials to be discarded and record the details in
Annexure-I.
5.4 CLEANING OF CHECKWEIGHER:
5.4.1 Switch off the Checkweigher and disconnect the main switch before cleaning activity.
5.4.2 Wipe the surface of Checkweigher with lint free mop.
5.4.3 Clean the surface of Checkweigher with 70% IPA solution.
5.4.4 Make the electrical connection and check its display.
5.4.5 After cleaning verify the Checkweigher and used.
5.4.6 Record the cleaning details in “Equipment Log” as per Format No
5.4.7 Frequency: Carry out the cleaning process every day before startup of a shift and at the end of Shift.
5.5 CALIBRATION OF CHECKWEIGHER:
5.5.1 Calibration Frequency: First working day of every month before use.
5.5.2 Check the cleanliness of the area.
5.5.3 Switch ‘ON’ the main power supply of the Checkweigher.
5.5.4 Turn the power switch 90 degrees clockwise to the “ON” the Checkweigher and check that zero is displayed on the screen.
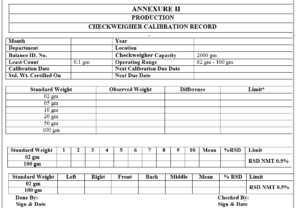
5.5.5 Place the Standard Weights one by one in the center of the Weighing Conveyor of the Checkweigher and note down the reading in the Annexure-II, Titled “Checkweigher Calibration Record”.
5.6 VERIFICATION OF CHECKWEIGHER:
5.6.1 Verification Frequency: Daily before use/immediately after maintenance work/Power Failure/Relocation of Checkweigher.
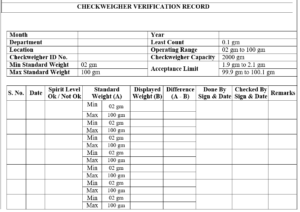
5.6.2 Verify the Checkweigher using two Standard Weights certified by Weights and Measures Department.
6.6.3 Place the Standard Weights one by one in the center of the Platform of the Weighing Conveyor and record the observations in Annexure-III, Titled “Checkweigher Verification Record”.
5.7 VERIFICATION OF DETECTED/REJECTED VIALS:
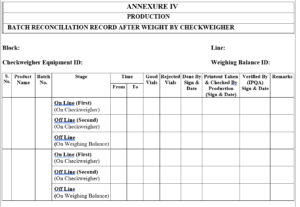
5.7.1 First time detected/rejected vials to be weighted again by pass through the same Checkweigher to detect the lower/higher weight.
5.7.2 Second time detected/rejected vials to be verified by using the weighing balance (off line) to detect the lower/higher weight.
5.7.3 Record the all above details in Annexure-IV, Titled “Batch Reconciliation Record after Weight by Checkweigher”.
5.7.4 Final rejection should be recorded in the Batch Manufacturing Record (BMR).
6.0 ABBREVIATIONS:
SOP Standard Operating Procedure
Ltd. Limited
QTY. Quantity
QA quality Assurance
7.0 ANNEXURES:
ANNEXURE No. TITLE OF ANNEXURE
Annexure-I Challenge Test Record For Checkweigher
Annexure-II Checkweigher Calibration Record
Annexure-III Checkweigher Verification Record
Annexure-iv Batch Reconciliation Record After Weight Check weigher
8.0 DISTRIBUTION:
• Controlled Copy No.1 Production Department
• Master Copy Quality Assurance Department
9.0 REFERENCES:
In House
Annexure-III
Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt