sop for Corrective and Preventive Action
1.0 OBJECTIVE:
1.1 To lay down the procedure for handling of Corrective and Preventive Action.
2.0 SCOPE:
2.1 This SOP is applicable for all Corrective and Preventive Action that are recommended if any, but not limited to the following documents:
2.1.1 Deviation
2.1.2 Out of specification (OOS)
2.1.3 Audit Report (Internal/External)
2.1.4 Trends analysis
2.1.5 Product recall
2.1.6 Market complaint
2.1.7 Incident Report
2.1.8 Annual Product review
2.1.9 Any other (Specify)
3.0 RESPONSIBILITY:
3.1 Quality Assurance:
3.1.1 To assign a number to the Corrective and Preventive Action (CAPA) form.
3.1.2 To issue the Corrective and Preventive Action form.
3.1.3 To follow up and close of the Corrective and Preventive Action after review.
3.1.4 To provide extension approval of the target date.
3.1.5 To ensure implementation of defined system.
3.1.6 To approve effectiveness of implemented CAPA.
3.2 Concerned Department Head:
3.2.1 To assign the Corrective and Preventive Action
3.2.2 To implement the Corrective and Preventive Action.
3.2.3 To complete the Corrective and Preventive Action as per defined schedule.
3.2.4 To check the effectiveness of implemented CAPA.
4.0 ACCOUNTABILITY:
4.1 Concerned Department Head/ Head QA
5.0 PROCEDURE:
5.1 Corrective and Preventive Action: A systematic approach that includes actions needed to correct (“correction”), prevent re-occurrence (“corrective action”) and eliminate the cause of potential nonconforming product and other quality problems (preventive action).
5.1.1 The CAPA is an event resulted from a system, process or procedure that may affect:
5.1.2 Safety, purity, potency or effectiveness of the product.
5.1.3 Health and safety of employees or customer.
5.1.4 Traceability of records.
5.2 The CAPA handling process is divided as follows:
5.2.1 Identification of non-conformance or failure
5.2.2 Reporting of non-conformance or failure.
5.2.3 Investigation of nonconformance or failure.
5.2.4 Corrective actions.
5.2.5 Preventive Actions.
5.3 Definitions of the key components of the CAPA are listed below
5.3.1 Corrective Action
It can be considered as remedial action for an existing problem after being a formal investigation or a complete resolution about non-conformances in products or systems.
5.3.2 Preventive Action
It can be considered as a proactive approach to prevent problems after being a formal investigation or a complete resolution about potential non-conformances in products or systems.
5.4 Failure
5.4.1 The inability of a component, intermediate or finished product to meet its specifications or its intended use, either before or after distribution of the finished product.
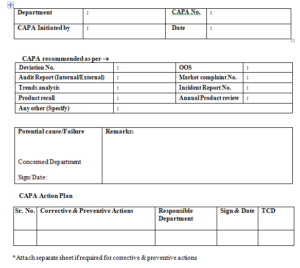
5.4.2 After review of approved documents (i.e. Deviation, , OOS reports ,Audit report (Internal/External) , trend analysis reports, Product recall, Market complaints, incident report, annual product review, Any other (specify), wherever Corrective and Preventive Action shall be filled in CAPA action form as per (Annexure –II). This CAPA action form shall have following details.
5.4.3 Department: The department which is initiating the CAPA.
5.4.4 CAPA No. : CAPA Number shall be entered in CAPA form.
5.4.5 CAPA initiated By: The Name of person who is initiating the CAPA.
5.4.6 Date: The date on which the CAPA is assigned.
5.4.7 CAPA Recommended: The Source due to which CAPA is initiated.
5.4.8 Potential cause/failure due to which CAPA is initiated.
5.4.9 CAPA action plan.
5.4.10 Verification & Closure of CAPA action plan.
5.4.11 Effectiveness verification of CAPA after implementation of Corrective & Preventive actions.
5.5 For each document a separate CAPA number shall be assigned for traceability and tracking.
A typical numbering system shall be:
Note : If reason of CAPA for Different documents is same then one common CAPA No. can be issued.
5.5.1 First two digits shall be the number for current year followed by a slash (/). (Like 18 for year 2018 and 19 for year 2019 and so on…).
5.5.2 Next three digits shall be the serial number beginning with 001 and shall start with 001 from 1st January of each year.
5.5.3 For example: First CAPA for the year 2023 was taken by Liquid and Ointment of abc Pharma Pvt. Ltd., Roorkee which shall be numbered as 23/001. Where 23 is the current year and 001 is the serial number assigned to Liquid and Ointment. The CAPA number for each fresh year shall start with the change in the year digit.
5.6 Potential cause/Failure for CAPA initiation
5.6.1 Mention the details of potential cause/failure in Annexure-II as follows
5.6.2 If it is from deviation mention deviation number with detail.
5.6.3 In case of audit mention audit remark and date of audit
5.6.4 In case of document review mention the document No.
5.6.5 In case of trend analysis, mention subject of trend analysis and its period.
5.6.6 In case of product recall, mention word recall followed by product and batch number.
5.6.7 In case of OOS mention OOS number.
5.6.8 In case of market complaint mention market complaint number.
5.6.9 In case of incident mention the incident.
5.6.10 In case of Annual product review mention the product name and year of review of the APR.
5.6.11 In case of other mention relevant details.
5.7 Corrective & Preventive Actions (CAPA):
5.7.1 CAPA shall be initiated and documented by concerned department designee.
5.7.2 QA manger shall evaluate the CAPA and shall approve the CAPA by signature.
5.7.3 Corrective & Preventive action target completion date shall be mentioned in CAPA action plan.
5.7.4 After completion of CAPA action plan, corrective & preventive actions shall be verified by Concerned Department & QA Manager.
5.7.5 Closure of the CAPA action plan shall be done by concerned department head, Plant Head/Director & QA Head.
5.7.6 If the corrective action involves a sub-contractor or supplier they should be notified and consulted on the proposed corrective action.
5.7.7 Based on investigation time line for CAPA shall be decided. CAPA shall be closed within 30 days or as per the date recommended by Head QA with proper justification. Any extension of the target date along with reason shall be mentioned by concerned department person along with comments from QA Manager.
5.7.8 The preventive action shall be recorded which includes but not limited to:
5.7.9 Training to the personnel.
5.7.10 Implementation of the actions.
5.7.11 Documentation of the actions taken.
5.7.12 CAPA effectiveness shall be checked after 3 months from closure of CAPA and Dept. Head shall give his/her comments for its effectiveness which shall be Verified by Plant Head/Director and Approved by QA Head.
5.8 Follow up (if required)
5.8.1 All CAPA shall be closed with proper documentation.
5.8.2 CAPA (corrective and preventive action) format shall be issued as per Annexure-II and its issuance record shall be maintained as per Annexure-I.
5.8.3 Flow chart for CAPA shall be defined as per Annexure-III
6.0. ABBREVIATION:
| S. No. | Abbreviations used | Full form of Abbreviation used |
| 1.0 | SOP | Standard Operating System |
| 2.0 | QA | Quality Assurance |
| 3.0 | CAPA | Corrective action and Preventive actions
|
| 4.0 | No. | Number |
| 5.0 | QC | Quality Control |
| 6.0 | NA | Not Applicable |
| 7.0 | TCD | Target Completion Date |
7.0. ATTACHMENTS (ANNEXES):
Annex – I : Corrective and preventive action form issuance record
Annex – II : Corrective and preventive action form.
Annex – III : Flow chart for CAPA
8.0. REFERENCE :
| S. No. | Reference Title |
| 1.0 | Pharmaceutical Quality System Q10
|
Annex – I
Corrective and preventive action form issuance record

Annex – II
Corrective and preventive action form.

Annex – III
Flow chart for CAPA

preparation for sampling Intimation slip
Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt
sop for Cleaning and operation of spray gun and assemble
sop for Fogging in Aseptic and Non Aseptic Area
Preparation of Site Master File
sop for Corrective and Preventive Action