sop for in-process control on liquids orals
1.0 OBJECTIVE
To ensure quality of product during Washing, Filling, Sealing, Inspection, Labeling and packaging operation of Liquid Orals.
2.0 RESPONSIBILITY
Quality Assurance Officer
3.0 ACCOUNTABILITY
Quality Assurance Manager.
4.0 PROCEDURE
4.1 START UP
Washing / Filling / Sealing and Inspection
4.1.1 Ensure that the relevant BMR is filled up to the previous stage and available in the Liquid
Filling area.
4.1.2 Ensure that the relative humidity and temperature of the Liquid filling area is within the limits prescribed in the Manufacturing Instructions
4.1.3 Ensure that the status tag , giving details of the Product to be filled and Batch Number is affixed on the machine.
4.1.4 Ensure that the Batch Number of the Bulk is the same as mentioned in the BMR.
4.1.5 Ensure that the Vessels containing Bulk have ‘APPROVED’ label on them.
4.1.6 Check the In Process Control Chart and ensure that the Production Officer has filled in the relevant columns giving product details.
4.1.7 After all the physical parameters have been set and the machine is in operation, collect sample and check
the physical parameters as per respective GPs.
4.1.8 Check the following parameters as per their respective GPs and record it in the In process Control Chart at
the start of operation and at random intervals not exceeding 2 hours.
Cleanliness of the bottles
Fill volume
Capping / Torqueing
Sealing / Leak test
4.1.9 The sample drawn for In process inspection shall be destroyed after performing the test.
4.1.10 In case the above parameters are not being met with, inform the Production Officer about the defect(s) noticed.
4.1.11 Production should start only after the defects have been rectified and certified by the Production Officer.
4.1.12 Recheck all the parameters and let Production continue in case the parameters are within the limit.
NOTE:- In case of any major defect or defects of recurring nature, immediately inform the Quality Assurance
Manager and ensure that the complete lot is quarantined.
4.2 Labeling & Packaging
4.2.1 On receipt of “Packaging Line clearance / Packaging Line Operation & Inspection record from the Production,
stating that the line and area is ready for Quality Assurance Inspection, inspect the following for cleanliness
and presence of any remnants of previous products / batch.
A, Turn table.
B, Labeling machine.
C, Packing belt and surroundings.
4.2.2 If the area is free from the previous product / batch, enter the same in Packaging Line clearance &
Packaging Line Operation & Inspection record and give the clearance.
4.2.3 Ensure that the details of packaging operation are indicated on the display board.
4.2.4 Ensure that the documents in BMR are duly completed upto the last operational stage.
4.2.5 Ensure that all the packaging components are on the line as per the packaging Order.
4.2.6 After the coding stereos are assembled and the coded Label is approved by production Officer, check the
coding of the stereo and ensure that coding details are as per the requirement of the product /pack / market with
respect to Batch No. , Mfg. Date , Exp. Date , M.R.P.etc. If the coding is correct, approve the same by signing on
the Label and attach in the Batch Manufacturing Record.
Note: Coding approval on Labels to be done for every day or whenever there is any change in the
stereos, operator / shift etc.
In case of off line coding ensure that all precoded packaging components are approved by production Officer and
the specimens are attached in the BMR. Quality Assurance Officer shall approve all the packaging components
and details prior to start of packaging operation.
4.2.7 Challenge the safety mechanisms fitted on machines and ensure their satisfactory working and record
the observation in Packaging line operation & inspection record.
4.2.8 Inspect the packs at the start of operation and at regular intervals (not exceeding 2 hours) as per
the sampling plan and record the observation in Packaging line operation and inspection record for the following.
a. Coding and printing details on Shippers ,Cartons & Labels.
b. Missing Label.
c. Crumpled / Slanted / Stained on labels
d. Inks lifting on Labels.
e. Empty Bottle.
f. Low fill / High fill Bottle.
g. Dented / Stained /Scratch marks on the bottles .
h. Scarting / Bridge breaking.
i Defective colures.
J Abnormal colour, odor & appearance.
4.2.9 In case any defect is observed inform immediately the Production Officer / Manager to rectify it. Record
the observations in the Packaging Line Operation and Inspection Record. Ensure that the defect has been rectified by the concerned Production personnel.
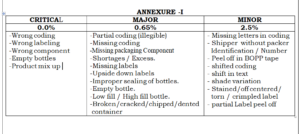
4.2.10 Classify the defects observed as per the guideline given in annexure-1.
4.2.11 In case any critical defect is observed the operation shall be immediately stop and the involved quantity
shall be quarantined. Operation shall be restarted only after taking corrective action.
4.2.12 In case of major and minor defects corrective action shall be immediately taken on the packaging line.
At the end of operation if the number of defective of one kind exceeds the acceptance number
Given in the sampling plan, the involved quantity shall be rejected and subjected for reinspection or necessary corrective action.
4.3 Ensure that the rejected packs are stored in containers with batch identity and rejection status label
of recoverable or non recoverable and send for further processing as per the Respective SOP.
4.4 Collect control & stability samples (Wherever required) of packed products. Record the details in Packaging
line Operation and Inspection Record and Control / Stability sample Register.
5.0 ABBREVIATIONS :
BMR = Batch manufacturing record.
MRP = Maximum retail price.
SOP = Standard Operating Procedure.
6.0 ANNEXURES :
ANNEXURE –1 : List of category of defects
Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt