sop for in process controls on tablets capsules packaging line
1.0 OBJECTIVE:
The objective of this SOP is:
1.1 To describe the procedure for monitoring the Quality of products during
blister or strip sealing, bulk filling and packaging operation of capsules or tablets.
2.0 RESPONSIBILITIES
2.1 Quality Assurance Officer shall be:
2.1.1 Responsible for assuring the quality of product during packing operations.
2.1.2 Responsible for checking and compliance of in-process parameters.
3.0 ACCOUNTABILITY:
Head – Quality Assurance
4.0 PROCEDURE:
4.1 Line Clearance
4.1.1 On receipt intimation for “Packaging Line clearance” from the production, check for line clearance as per SOP
4.2 Blister / Strip Sealing Operation
4.2.1 Ensure that the material of correct product / batch is brought to the blister or strip sealing room.
4.2.2 Ensure that details about operation are indicated on equipment label.
4.2.3 Ensure that the temperature and relative humidity of blister or strip packing room is in accordance with the limit mentioned in the BMR and is recorded in environment control record by production personnel.
4.2.4 After the coding stereos are assembled and the coded foil is approved by production Officer, check the coding from each of the stereos and ensure that coding details are as per the requirement of the product or pack or market with respect to Batch No., Mfg. Date, Exp. Date, M.R.P. etc. if the coding is correct, approve the same by signing on the foil attach the foil in the Batch Manufacturing Record.
4.2.5 Carry out the leak test of blister or strips at beginning of operation and there after regular interval about two hours as per General Procedure
4.2.6 The samples drawn for leak testing shall be destroyed after performing the test as per SOP
4.2.7 Inspect the blister or strip pack at the start of operation and at intervals about two hours as per the sampling plan and record the observation in Packaging line operation and inspection records of BMR for the following.
a. Coding and printing details
b. Knurling on strips or blisters
c. Blister formation.
d. Cutting edges of blisters or strips.
e. Inks lifting on foils.
f. Punctured and empty pockets.
g. Scratch marks and spots on blisters or strips.
h. Powder shedding in capsules.
i. Telescopic and dented capsules or Capped, chipped or broken tablets (as the case may be).
j. Abnormal colour, appearance of tablets or capsules.
k. Improper locking and poor printing or embossing on capsules or tablets. (as the case may be).
4.3 Packaging Operations:
4.3.1 Ensure that the details of packaging operation are indicated on the display board.
4.3.2 Ensure that the material of correct product / Batch No. is brought
to the packaging line in suitable containers or pallets with identification labels.
4.3.3 Ensure that all the packaging components on the line are as per the packaging order.
4.2.12 In case of off line coding, ensure that all coded packaging components
are approved by production Officer and check the coding of the stereo
and ensure that coding details are as per the requirement of
the product or pack or market with respect to Batch No., Mfg.
Date, Exp. Date, M.R.P. etc. If the coding is correct, approve
the same by signing on the packing component and attach in
the Batch Manufacturing Record. Check and sign in the
reconciliation document of off line coding packing material.
4.3.4 Check the packaging operation at the start and then at interval about
two hours as per sampling plan Check for the following.
a. Coding details on blisters or strips, catch boxes, unit cartons, outer cartons shipper and shipper labels etc.
b. Quantity in boxes, unit cartons, and outer cartons and their arrangement.
c. Packaging components including pads, partitions, literature etc. and their arrangement.
d. Sealing of polybags (if applicable), BOPP tapping of inner or outer shipper.
e. Tilted labeling on unit cartons and shippers.
f. Any visible defects in blister or strips.
g. Text and colour of blisters or strips, catch boxes, unit cartons, outer cartons, shipper and shipper labels etc.
4.4 Bulk Packaging of Capsules or Tablets in Bottles
4.4.1 Ensure that the correct product or batch is brought to the bulk filling area.
4.4.2 Ensure that the temperature and relative humidity of the bulk filling area is in accordance with the limit mentioned in BMR and is recorded in environment control record by production personnel.
4.4.3 Ensure that the tablets have been de-dusted and sorted or the capsules have been sorted and polished (as the case may be).
4.4.4 Ensure that all the packaging components are as per the packaging order.
4.4.5 Check the filling operation at the start and then at interval about two hours as per the sampling plan. Check the following.
a. Coding details on the label of bottles.
b. Bottles and closures are free from dust, denting, black spots etc.
c. Packaging components like cotton wool, literature etc. (wherever applicable)
d. Torn label / literature.
e. Uncoded labels.
f. Observe for powder shedding, telescopic, denting, poor polishing, poor printing, locking defects for capsules Observe for capped, chipped, broken and improper printing or embossing on tablets.
g. Check for quantity of tablets or capsules by weight or by count.
4.4.6 Carry out the leak test of bottles where induction sealing is performed twice in a shift as per General Procedure.
4.4.7 The sample drawn for leak testing shall be destroyed after performing the test.
4.4.8 In case any defect is observed inform immediately the Production Officer to rectify it. Record the observations in the Packaging Line Operation and Inspection Record of BMR. Ensure that the concerned Production personnel have rectified the defect.
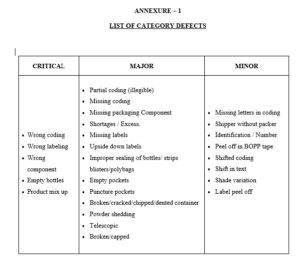
4.5 Classify the defects observed as per the guideline given in annexure-1.
4.5.1 In case any critical defect is observed the operation shall be immediately
stop and the involved quantity shall be quarantined. Operation shall be
restarted only after taking corrective action.
4.5.2 In case of major and minor defects corrective action shall be immediately
taken on the packaging line. At the end of operation if the number of defective
of one kind exceeds the acceptance number given in the sampling plan,
the involved quantity shall be rejected and subjected for re-inspection or necessary corrective action.
4.6 Ensure that the rejected blister or strips or bulk packs are stored in containers
with batch identity and rejection status label of recoverable or non recoverable
and send for further processing as per the Respective SOP.
4.7 Collect control & Stability samples (Wherever required) of packed products as per SOP Record the details in BMR and Control Sample and Stability sample Register.
5.0 REASON FOR REVISION:
This SOP is modified in order to be more effective in its responsibility, procedure and incorporation of schematic diagram (Refer Annexure – 2) for better presentation.
6.0 TRAINING:
Trainer — Head – Quality Assurance
Trainee — Quality Assurance Officers
7.0 DISTRIBUTION:
Certified Copy No. 1: Head of Department – Quality Control
Certified Copy No. 2: Head – Plant Operations
Original Copy : Head – QUALITY ASSURANCE
8.0 ANNEXURE:
Annexure – 1 : List of Category Defects
Annexure – 2 : Schematic Diagram
9.0 REFERENCE:
In-house
ANNEXURE – 2
SCHEMATIC DIAGRAM


Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt